MERIT’s spirometry and MBPC expertise form the cornerstone for successful Phase III bioequivalence study for generic asthma treatment
Situation
A large generic pharmaceutical company conducted a Phase III clinical trial to demonstrate the bioequivalence (BE) of its generic albuterol sulfate metered dose inhaler using methacholine bronchoprovocation challenges (MBPCs) in adult subjects with asthma.
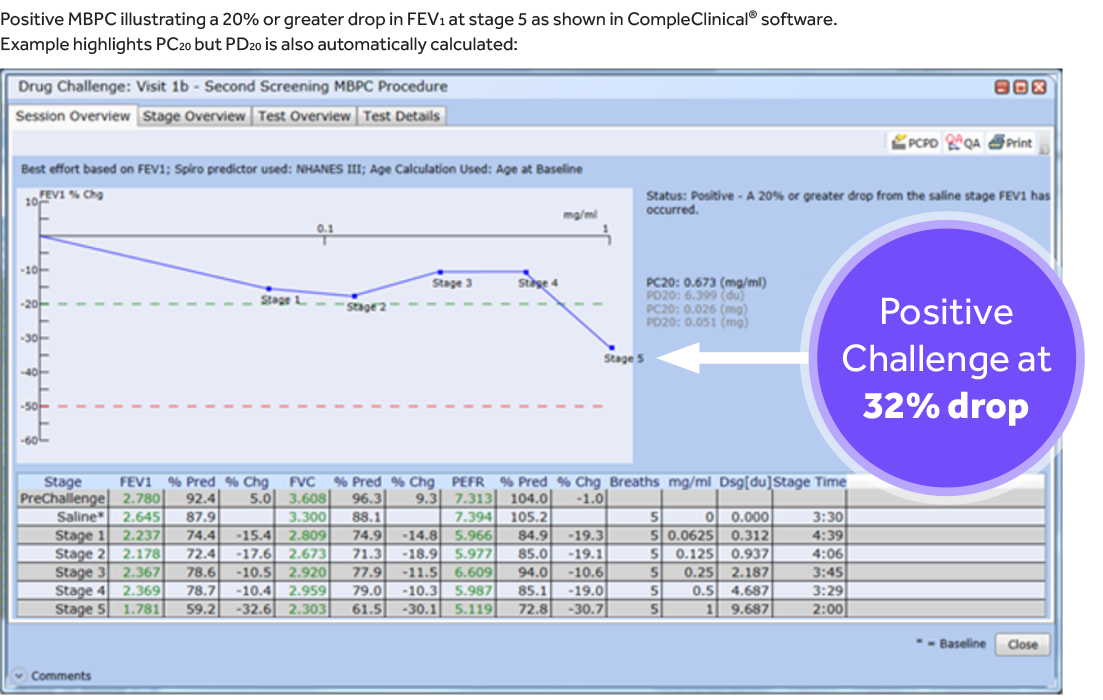
MBPCs are a dose-response test where subjects inhale an increasing dose of methacholine chloride solution via nebulizer, performing spirometry within a certain time frame after each dose, until they reach the provocative dose or provocative concentration of methacholine required to cause a 20% drop (PD20 or PC20) in the forced expiratory volume in 1 second (FEV1), which is considered a positive challenge.
“I have conducted many complex respiratory trials over the years, but there’s no doubt that MBPCs are some of the most difficult. Studies undertaken with this model require high-quality spirometry acquisition software and equipment. Even more crucial is having a CRO team with deep understanding of the challenge process.”
– BOARD-CERTIFIED ALLERGIST-IMMUNOLOGIST, STUDY PI
Challenges
Due to the complexity of MBPCs as well as stringent eligibility requirements, screening 217 subjects and completing 88 was an arduous process for this study. Subjects were required to complete two screening visits and five treatment visits for a minimum of seven MBPCs throughout the study.
The following potential hurdles had to be addressed:
- VARIABILITY IN SPIROMETRY DATA: When MBPCs are conducted across multiple sites, as is required for a pivotal BE study such as this, variability in the spirometry data that is the primary outcome measure for MBPCs can be introduced. In addition, spirometry is effort-dependent and requires skilled and consistent coaching.
- NON-STANDARD EQUIPMENT: Non-standard nebulizer, spirometry, and dosimeter equipment can also be a source of variability in the data.
- MEETING FDA GUIDANCE AND ATS/ERS STANDARDS: Another complexity of conducting this type of BE study is determining a workable protocol while integrating the FDA guidance on albuterol sulfate. The guidance also requires following ATS/ERS standards for spirometry and MBPCs.
- AGGRESSIVE STUDY TIMELINES: Very rapid study enrollment and randomization goals were set for the study as well as swift study closeout timelines.
- METHACHOLINE DILUTION PROCESS: Sourcing and diluting the methacholine required for this type of study can be a difficult process. Producing the correct number of frozen methacholine kits in a timely manner is vital, as methacholine degrades over time, eventually rendering it unusable.

Solution
The sponsor chose MERIT to be their centralized spirometry and MBPC partner for this project. We provided standardized spirometry and challenge equipment, certified site training, spirometry capture utilizing our userfriendly proprietary software, CompleClinical®, and meticulous data management over the course of the study.
- REDUCED SPIROMETRY DATA VARIABILITY THROUGH CENTRALIZED GRADING & REVIEW: MERIT’s team of Registered Respiratory Therapists (RRTs) provided essential oversight and training throughout the study. RRTs graded all maneuvers across every site to ensure that ATS/ERS guidelines were met. Centralized review allowed SMEs to track trends across sites, particularly in PC20FEV1, the primary endpoint of this study.
- STANDARDIZED EQUIPMENT: While various guidance documents on MBPCs allowed use of any characterized equipment, the success of the study was enhanced by using standardized equipment across all study sites. MERIT provided specific recommendations on the standardized equipment chosen, offering another important reduction in variability.
- EXPERIENCED STUDY TEAM TO CONSULT ON PROTOCOL DESIGN: While the FDA Draft Guidance on Albuterol Sulfate provides the minimum requirements for protocol design, MERIT’s familiarity with the model allowed our team to suggest refinements to the study design. These refinements can make the difference between failure and success. Optimizations included the number of Test and Reference doses, population enrichment strategies, and order of procedures.
- CENTRALIZED METHACHOLINE DILUTIONS: MERIT’s extensive knowledge of the entire methacholine dilution process provided efficient management of methacholine chloride solution dilution, testing, and distribution.
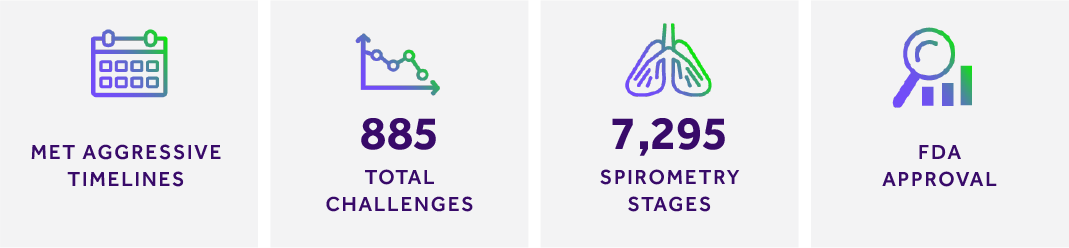
- SWIFT ENROLLMENT & STUDY CLOSEOUT ACTIVITIES: Due in part to MERIT’s extensive, personalized site training, all sites were released to screen within one week of the Investigator’s Meeting. In the first three weeks of the study, over 30% of the total subjects were enrolled. By week seven, we had reached our enrollment goal of 200 subjects. Enrollment was scheduled to take approximately four months; we reached our enrollment goals in less than nine weeks. Database lock occurred within 20 days of the Last Subject Completed. The project completed ahead of schedule and under budget.
“We just randomized our first patient for this study. This was completed just 1 week after we released sites to screen. This is a huge accomplishment for the team! I want to personally thank you for all your hard work on this study and ensuring we met this important milestone!”
– SPONSOR PROJECT MANAGER
“In comparison to all the other spirometry vendors we have worked with, MERIT is clearly above all the others! This is because EVERYONE in the company seems to be extremely familiar with all aspects of the protocol as it relates to the MERIT equipment and supplies. There have been numerous occasions where we have had to get urgent assistance from your help desk team when patients have been in the middle of their challenges. The help desk has always been able to respond immediately and solve the problems on the spot. This is not the standard of the industry and so appreciated by us.”
– STUDY SITE COORDINATOR
Results & highlights
- SPEED: MERIT supported rapid enrollment and study startup as well as swift study closeout.
- VOLUME OF CHALLENGES: There were 885 total MBPC sessions conducted, a complex and lengthy process, which relied on skilled and effective training provided by MERIT.
- NUMBER OF SPIROMETRY STAGES: MERIT graded 7,295 MBPC spirometry stages throughout the study, ensuring that spirometry was properly performed.
- FDA APPROVAL: With MERIT as the sponsor’s MBPC/centralized spirometry partner, the FDA approved the generic albuterol treatment.
“On behalf of our Inhalation group, many thanks to everyone for their hard work and dedication. This is a significant milestone for us, and I am delighted we could deliver for the company.”
SPONSOR VP, GLOBAL CLINICAL DEVELOPMENT
It’s important to have a tenured, expert team for complex study designs such as bioequivalence based on MBPCs. Studies undertaken with this model require high-quality spirometry data and a partner with a deep knowledge of the challenge process. With MERIT, your critical primary endpoint is safe in our hands.
Connect with us to learn more about how our expertise and approach can support bringing your product to market on-time and on-budget. Your success is our priority.
START A CONVERSATION
E: Info@meritcro.com | P: (608) 284-8810


