
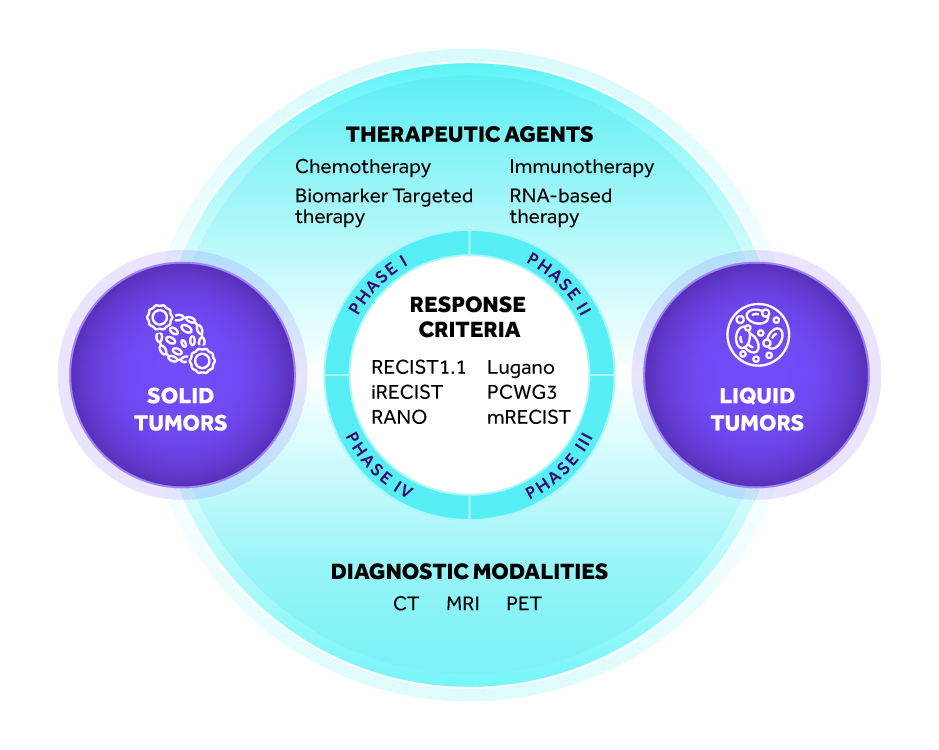
Therapeutic Expertise: Oncology
MERIT is a global clinical trial imaging endpoint services provider focusing on oncology studies. Our team are experts in managing and evaluating radiological images. MERIT’s knowledgeable clinical trial readers are board-certified to assure high-quality, consistent data review and interpretation.
With MERIT, you benefit
from the following:
- Comprehensive Imaging Services: Our oncology reading centers in the US, Europe, and China provide end-to-end central imaging services.
- Single System: Unlike others that depend on separate applications, our proprietary software, EXCELSIOR™, delivers automation in a single system for all image and workflow management that supports lesion segmentation with the corresponding response criteria such as RECIST1.1.
- Collaborative Partnership: We prioritize a collaborative, partnership-driven approach for all projects with scalable solutions and personalized attention. We adapt quickly to meet your needs. If you’re looking for an oncology imaging services provider that will value your partnership and make your study a priority, consider MERIT.
ONCOLOGY SERVICES
IMAGING SERVICES:
- Protocol Design & Consultation
- Site Certification, Management, & Support
- Image Upload, QC, & Storage
- Image Read & Adjudication
- Data Management
- Regulatory Support
MERIT has provided image collection, centralized reading, and data management services for clinical trials since 2012. Using our innovative and proven technologies and intuitive, seamless workflows, MERIT’s experienced staff bring more than a decade of clinical endpoint expertise to ensure the success and integrity of your independent imaging review studies.
- Single platform for all study data, queries, reporting/tracking, image transfer and analysis
- Oncology staff averages 10+ years clinical trial experience and many have advanced degrees
- Step-by-step Imaging Protocols, SOPs, and document support
- Indispensable regulatory support and guidance
- Established, time-tested Quality Assurance, security, and data privacy
MERIT’S READER SERVICES
Expert Readers for Clinical Trials
Our readers are board-certified radiologists experienced in performing radiology assessments for clinical trials in a variety of indications.
Response Criteria & Indication
Our readers are trained in response criteria, measurement requirements, and software functionality.
Custom Service Offerings
All readers will be trained for study-specific reading considerations according to each study charter and sponsor requirements.
COLLECT AND HOLD SERVICE
MERIT’s Collect and Hold services are designed to support early phase oncology clinical trials through the prospective collection and quality control of imaging data, that is – getting study images to a “ready-to-read” state. This is made possible by our streamlined, end-to-end software platform, EXCELSIOR™.
Collect and Hold services include image collection and quality check, site support, and project management services.
This service provides clinical trial sponsors and research organizations with several benefits, including:
- Cost Savings
- Accelerated Drug Development Timelines
- Improved Image Quality
- Reduced Variability

IMAGING SOFTWARE
EXCELSIOR is the only integrated, market-leading platform designed for managing clinical trials that offers a clear advantage across image management, the read process, and response outcome reporting. Our all-in-one platform supports clinical trial management experts, site technicians, and oncology reading centers, providing end-to-end processes with clean, complete, and accurate data to meet any clinical trial needs.
EXCELSIOR is a 510(k) cleared medical device for radiological indications that is HIPAA compliant and meets all 21 CFR Part 11 requirements for electronic records and signatures.
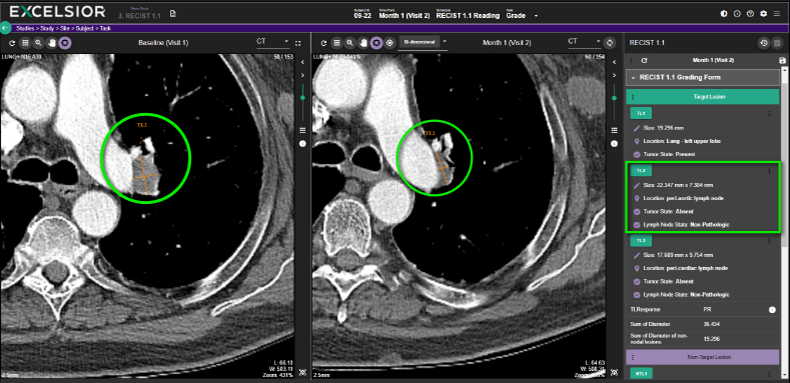
LESION REPORTING PROCESS
EXCELSIOR enables viewing DICOM images in split screen across study timepoints to measure and compare lesions qualitatively and quantitatively.
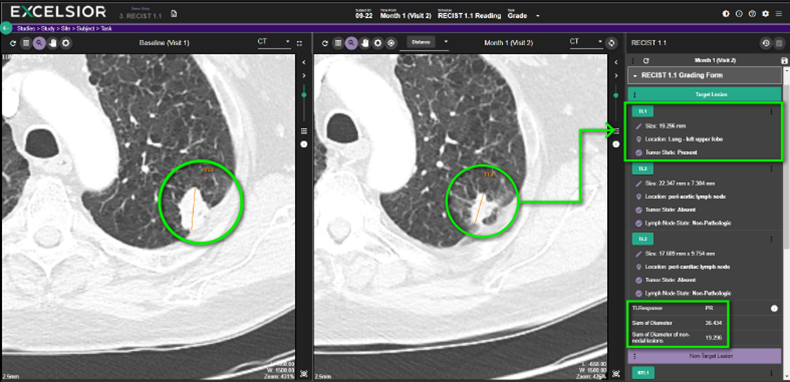
EXCELSIOR image viewer interactively records measurements, qualitative assessments, and anatomical location to generate calculated outcome/responses, comparing values across timepoints.
KEY EXCELSIOR FEATURES
EXCELSIOR is a time-saving and validated image analysis platform. Our software platform offers many features that help support the read process to minimize the risk of bias and to provide accurate and robust endpoint data for your clinical trial.
- Efficient and accurate image analysis platform supports efficient reader workflow
- Ability to configure and optimize study workflows with the corresponding response criteria
- Minimizes the risk of bias assessment
- Streamlined platform to manage subjects and data
Accurate analysis and proven solutions with MERIT.
A successful multi-center trial requires intrinsic understanding of various imaging techniques, developing meaningful outcome correlations, and formulating computational methodologies. Our insightful experts help guide your selection of the right biomarkers that accurately reflect the characteristics of disease progression and treatment effects.
Some companies tend to overpromise and underdeliver, which puts your study success at risk. If you’re looking for an oncology services provider that will value your partnership and make your study a priority, consider MERIT.
ONCOLOGY EXPERTISE
MERIT provides added value to complex clinical trial projects through personalized service, deep scientific expertise, and exceptional quality. Our team includes:
Our team has extensive knowledge and experience in the imaging endpoint process standards of clinical trials. MERIT’s Quality Assurance team continuously monitors and maintains quality, providing crucial regulatory support through detailed SOPs and QA documentation. All MERIT staff are trained in GxP, human subject data protections, and GDPR readiness.
LEVERAGING OUR GLOBAL EXPERIENCE

We provide the same meticulous attention to detail for your clinical trial workflow whether you’re a biotech sponsor just launching your pipeline, or you’re an established pharmaceutical sponsor. Our refined and standardized processes from our global ophthalmology experience support efficiencies in our services across therapeutic areas. This often positively reduces overall costs and shortens timelines for our sponsors and partners.

