MERIT’s spirometry and MBPC expertise form the cornerstone for successful Phase III bioequivalence study for generic asthma treatment....
Inhale Confidence. Exhale Delays.

Inhale Confidence. Exhale Delays.
Don’t let respiratory data slow your clinical trial. Partner with MERIT for a seamless, data-driven approach. Our seasoned project managers and rigorously trained RRTs ensure meticulous data quality throughout your entire study.
MERIT offers a customizable, turnkey solution tailored to your specific needs. Breathe easy with exceptional customer service every step of the way.

“MERIT was a very valuable partner in our phase 2/3 program as the central spirometry vendor. The oversight they provided to this important component of the program was a key factor in its success. To not use a vendor such as MERIT in a large respiratory program would make executing the studies much more difficult and greatly limit our confidence in the spirometry data.”
-SPONSOR, LEADING BIOPHARMACEUTICAL COMPANY
MERIT’s standardized collection and consistent interpretation of respiratory data enhances accurate, actionable outcomes. By centralizing your spirometry overreads with MERIT, you can be sure your data is in the hands of qualified respiratory therapists who are committed to personalized service. MERIT offers reliable solutions and a responsive partnership for Phase I-IV, pharmacokinetic, and bioequivalence respiratory clinical studies.
What if you could fully-engage sites in every stage of a respiratory clinical trial from startup to conductance to closeout so that your next study completed ahead of time and under budget? Take a look at a few metrics from one of our methacholine challenge bioequivalence studies.

SPIROMETRY SIMPLIFIED. Our qualified respiratory therapists can be contacted with one simple phone call or email. Sites get the swift assistance they need without waiting on hold or for a return phone call that never comes. Sponsors benefit with improved subject qualification and retention at screening and throughout the study.
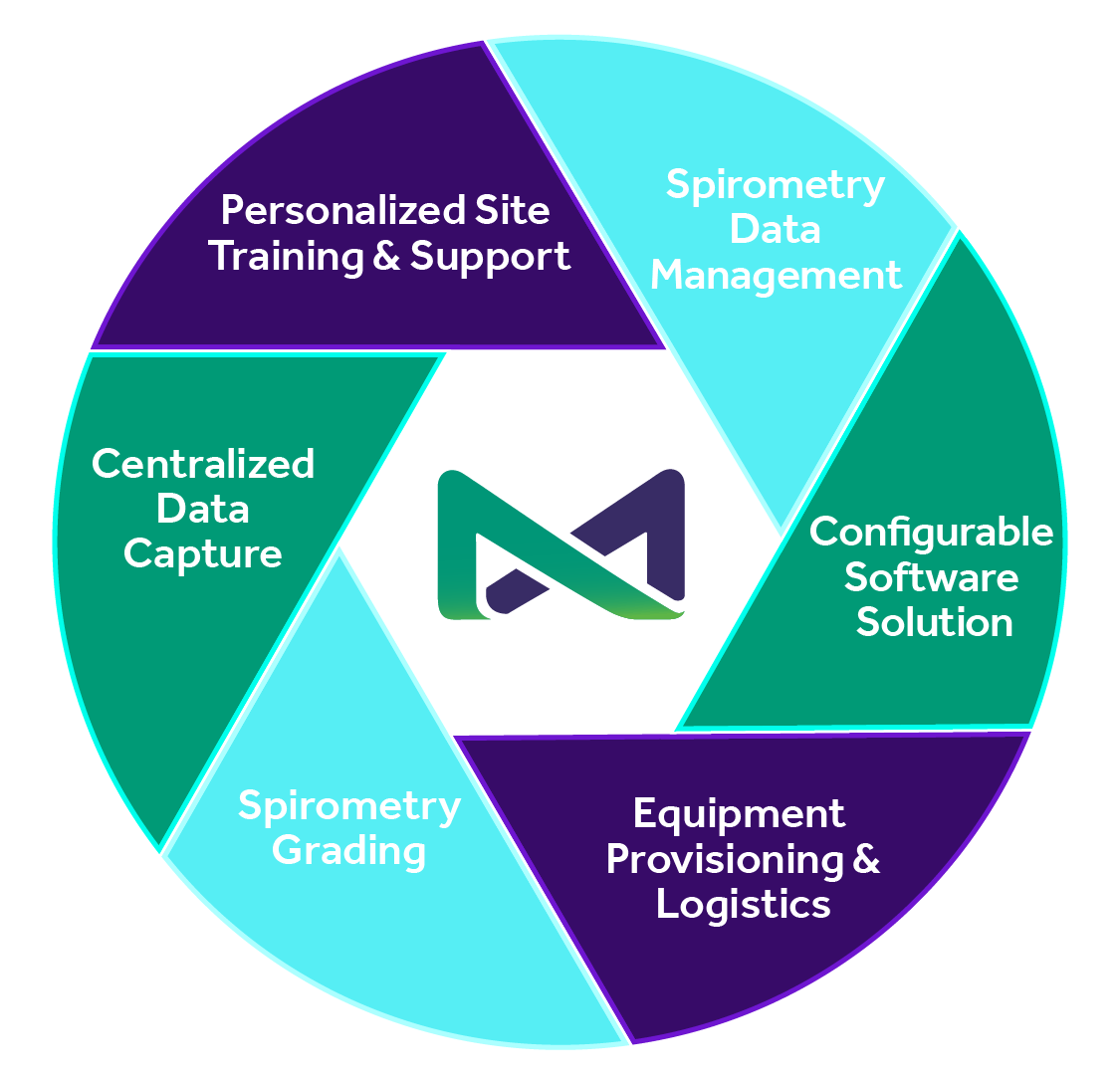
Whether studying a novel treatment for COPD or performing a generic bioequivalence study for asthma, most pulmonary clinical trials include spirometry as a primary endpoint. Our expertise in centralized spirometry supports the actionable results required to move your trial forward, and includes:
Centralized spirometry overreads by Registered Respiratory Therapists (RRT) team using configurable, standardized grading to confirm & adhere to protocol and ATS/ERS standards
Uniformly sourced and rigorously tested equipment with study-specific manuals provided to each site
Proprietary spirometry software enables real-time acquisition of data, empowering faster quality control and a more efficient site query process
Hands on training for all users with clear instructional materials including manuals, videos, and webinars

MERIT’s spirometry and MBPC expertise form the cornerstone for successful Phase III bioequivalence study for generic asthma treatment....
MERIT’s spirometry expertise and quality overreads lay the foundation for successful multi-study COPD drug development program....
MERIT’s innovative software application, CompleClinical®, simplifies and streamlines the respiratory clinical trial process, improving the efficiency and accuracy of data capture, management, and grading of spirometry data. Our software features highly customizable workflows that help facilitate rapid study startup and enrollment. CompleClinical provides audit trail tracking and is HIPAA and 21 CFR Part 11 compliant.

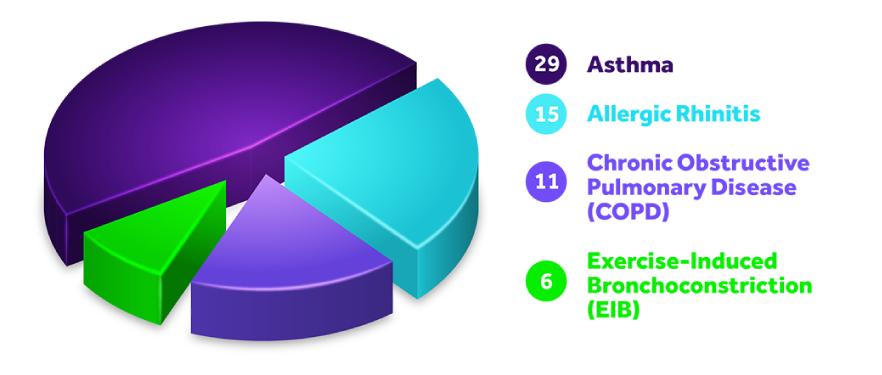
ASTHMA
Asthma is a chronic disease in which the airways in the lungs narrow and swell, causing labored breathing, wheezing, chest tightness, and cough.
COPD
Chronic Obstructive Pulmonary Disease (COPD) is a group of progressive lung disorders characterized by increasing breathlessness including emphysema and chronic bronchitis.
ALLERGIC RHINITIS
Allergic rhinitis is an allergic response to environmental allergens such as pollens, weeds, dust mites, pet dander, insects, and molds that causes sneezing, nasal congestion, itchy nose, and sore throat.
EIB
Exercise-Induced Bronchoconstriction (EIB) is a temporary narrowing and inflammation of the airways of the lung due to physical activity. Symptoms include shortness of breath, wheezing, coughing, and chest tightness.
CYSTIC FIBROSIS
Cystic fibrosis is an inherited life-threatening disease caused by a defective gene that affects cells that produce mucus, digestive juices, and sweat. It is a progressive disease that damages the lungs, pancreas, and other organs.
IDIOPATHIC PULMONARY FIBROSIS
Idiopathic Pulmonary Fibrosis (IPF) is a progressive and irreversible lung disease characterized by the development of scar tissue (fibrosis) in the lungs. The term “idiopathic” means that the cause of the condition is unknown. IPF is categorized as a restrictive lung disease due to the decrease in lung elasticity. Scarring of the lung tissue makes the lungs become stiff, therefore expanding less, and decreasing overall lung function and oxygen update.

