MERIT’s spirometry expertise and quality over-reads lay the foundation for successful multi-study COPD drug development program
Situation
A leading biopharmaceutical company developed a once-daily, nebulized long-acting muscarinic antagonist (LAMA) innovator treatment for moderate to severe chronic obstructive pulmonary disease (COPD). The drug development program included:
- 2 Phase II dose-ranging studies
- 3 Phase III pivotal safety and efficacy trials
Challenges
Primary endpoints for the studies were trough FEV1 at the end of the various treatment periods, as demonstrated by serial spirometry at numerous timepoints (for example, at 2, 4, 6, 8, 16, and 24 hours after drug administration). The following challenges arose for each of the five trials:
- INCREASED VARIABILITY: Spirometry is effort-dependent, which introduces the risk of increased variability and requires skilled and consistent coaching
- COMPLEX ATS/ERS STANDARDS: FDA guidance almost always requires adherence to lengthy, complex ATS/ERS spirometry standards, including criteria for repeatability, usability, and acceptability
- NUMEROUS SPIROMETRY TIME POINTS: Consistent and efficient grading of numerous time points for serial spirometry is demanding. With up to 7 stages (3-8 efforts per stage) within a 24-hour period, grading this many efforts can prove extremely difficult, even for the most tenured respiratory experts
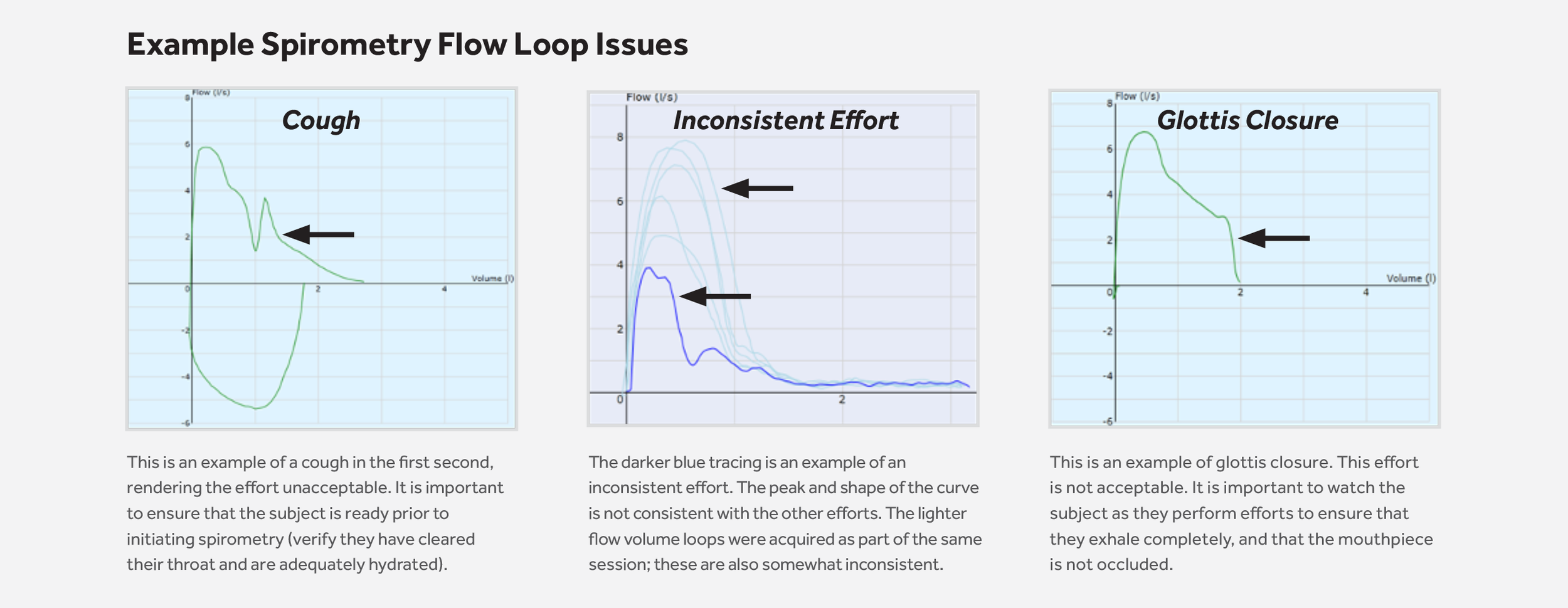
- FLOW LOOP ISSUES: Spirometry flow loops require careful examination by a protocol-trained Registered Respiratory Therapist (RRT) to recognize potential problems in the effort
Solution
The sponsor chose MERIT to be their centralized spirometry partner for all five clinical trials for their LAMA innovator treatment for COPD. We provided standardized equipment, certified site training, spirometry capture utilizing our user-friendly proprietary software, meticulous data management and hundreds of thousands of high-quality spirometry overreads over the course of four years.
- QUALITY OVERREADS: Given that spirometry was a primary endpoint in all five studies, the quality of the spirometry overread service was paramount. MERIT’s team of RRTs ensured all spirometry met ATS/ERS standards. They delivered efficient and repeatable overreads leading to less variable FEV1 data suitable for proving drug efficacy and ultimately, FDA submission and approval
- EXPERTISE: The MERIT respiratory team had a solid understanding of the specific nuances of a COPD drug trial as well as how to manage concurrent large, centralized spirometry trials
- STANDARDIZED EQUIPMENT & TRAINING: Uniformly sourced and rigorously tested equipment were provided along with study-specific manuals. Hands on training was presented at Investigator Meetings and supplemental training included comprehensive videos and webinars
- SPEED: The Phase II studies were completed much faster than initially expected and included accelerated timelines for data acquisition, database lock, and transfer of the final data. MERIT exceeded expectations by providing the validated final data transfer 17 days ahead of schedule for one study and 39 days ahead of schedule for the other study
- CLEAN FINAL DATA SET: The cleaning and validation of the final data set was completed in a timely manner and delivered, ready for FDA submission
“MERIT was a very valuable partner in our phase 2/3 program as the central spirometry vendor. The oversight they provided to this important component of the program was a key factor in its success. To not use a vendor such as MERIT in a large respiratory program would make executing the studies much more difficult and greatly limit our confidence in the spirometry data.”
— SPONSOR, LEADING BIOPHARMACEUTICAL COMPANY
Results & highlights
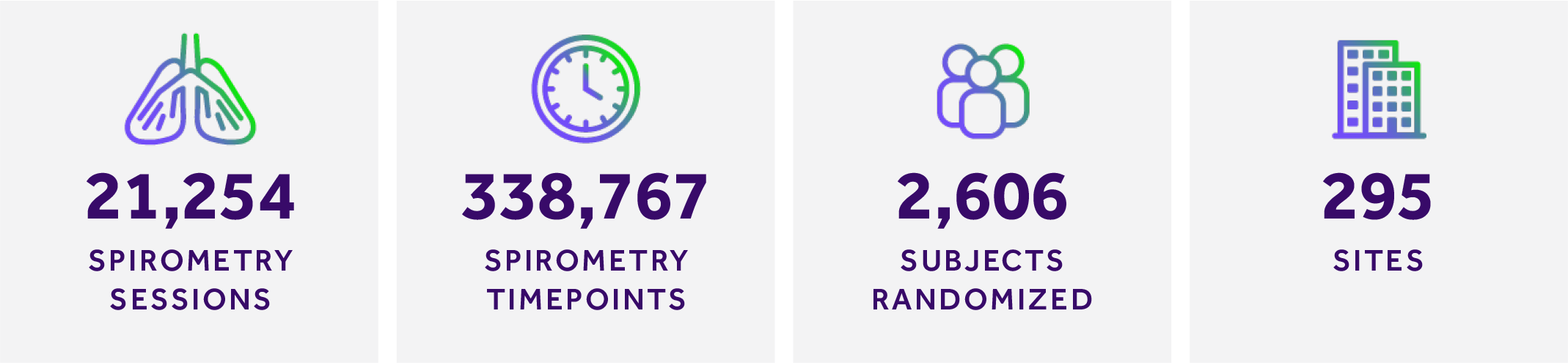
- VOLUME OF SPIROMETRY TIMEPOINTS: MERIT graded over 338,000 individual timepoints for spirometry over the five studies
- FAST TURN AROUND TIME: There were over 21,000 spirometry sessions for the program with an average turn-around time for overreads of less than 6 hours
- STUDY START UP EFFICIENCES: Over 2,500 subjects were randomized at 295 sites over the course of the four-year program. Efficiencies were gained by MERIT’s strong relationships with trusted sites, accelerating study start up times due to the sites’ familiarity with software and equipment
- FDA APPROVAL: With MERIT as the sponsor’s centralized spirometry partner, the FDA approved the LAMA innovator treatment
It’s important to have a tenured, expert team for complicated spirometry overreads to spot potential issues with flow loops and provide necessary oversight. With MERIT, your critical primary endpoint is safe in our hands.
Connect with us to learn more about how our expertise and approach can support bringing your product to market on-time and on-budget. Your success is our priority.
START A CONVERSATION
E: Info@meritcro.com | P: (608) 284-8810


