Our Info Sheets are all downloadable and include helpful checklists, challenge and solution guides, and additional brief cheat sheets.

Info Sheets

How Equipment Certification Enhances Image Quality
Equipment certification plays a crucial role in maintaining and improving image quality in clinical trials. Learn how it impacts the overall quality of imaging in our info sheet.

Making the Leap to a New Clinical Endpoint Expert
Deciding it’s time to engage a new clinical endpoint service provider is never easy, but sometimes it’s necessary. Your current provider may not have the experience or scalability you need to move your programs ahead.

Endothelial Cell Count (ECC) Analysis
Endothelial Cell Count (ECC) is an important measurement to evaluate corneal endothelial cell health. MERIT provides masked, independent ECC reading, as required in many ocular clinical trials.

Why FDA 510(k) Clearance Matters
Not all technology platforms are created equal. MERIT’s EXCELSIOR™ system not only increases accuracy and efficiency by providing a suite of advanced endpoint analysis tools, but it also provides the regulatory assurance of FDA 510(k) clearance. Learn what benefits FDA 510(k) clearance offers in our info sheet.

Unlock Confidence in Your Clinical Trials with MERIT
MERIT’s ISO 13485 certification of our Quality Management System provides more than just regulatory compliance. It demonstrates our commitment to quality, safety, efficiency, and consistency. Read our info sheet to learn the advantages of collaborating with an ISO 13485 certified company like MERIT.

EXCELSIOR: Increasing Efficiency in Oncology Clinical Trials
MERIT’s cloud-based EXCELSIOR software platform enables easy upload of oncology images (CT, MRI, PET) to one secure, validated and FDA-cleared application. This facilitates real-time, trackable, and transparent communication. Learn more in our info sheet.
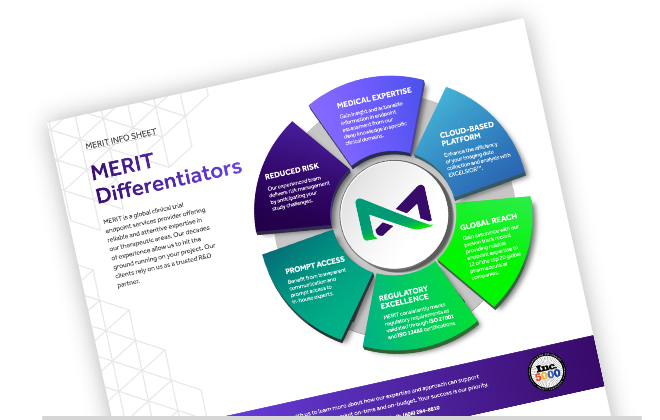
MERIT Differentiators
MERIT is a global clinical trial endpoint services provider offering reliable and attentive expertise in our therapeutic areas. Learn more about what sets MERIT apart in this info sheet.

Challenges and Solutions for Gene Therapy Trials in Inherited Retinal Diseases
Gene therapy trials in Inherited Retinal Diseases (IRDs) present unique challenges for biopharma drug developers. Choosing MERIT as your clinical endpoint expert gives you access to solutions based on experience supporting 31 gene therapy trials in IRDs.

6 Steps for Selecting Ophthalmology Safety Endpoints in Non-Ophthalmology Clinical Trials
What if your company’s early meetings with the FDA to review your preclinical data have indicated a need for ophthalmology safety endpoints in your upcoming non-ophthalmology clinical trial? Discover what your next steps should be in this info sheet.

CRO Partnerships with MERIT
Many full-service CROs have elected to integrate and use our solutions to manage the imaging and data management elements of their clinical studies. We are thoroughly familiar with the hurdles that CROs may face and can help you proactively address them to meet your customers’ expectations. Learn about the benefits of CRO partnerships with MERIT.

Four Cornerstones of Merit Services
MERIT’s commitment to quality is the foundation of our safe, reliable, and efficient data analysis and management services for clinical trials. Discover the four cornerstones of MERIT services in this info sheet.

Exceptional Reading Center Services for Global Phase III Wet AMD Study
MERIT’s ophthalmic expertise and cloud-based imaging platform performed a crucial role in a global Phase III Wet AMD study. Take a look at a few key metrics from our case study.

Importance of ETDRS BCVA in Ophthalmic Trials
ETDRS BCVA has become the gold standard for BCVA testing and plays an integral role in ophthalmic clinical trials. Discover 3 vital aspects of ETDRS BCVA in our info sheet.

Site Engagement Infographic
What if you could fully-engage sites in every stage of a respiratory clinical trial from startup to conductance to closeout so that your next study completed ahead of time and under budget? Take a look at a few metrics from one of our methacholine challenge bioequivalence studies.

How Does Site Support Impact Respiratory Trials?
Learn how site support and engagement leads to faster recruitment, better retention, and shortened timelines for respiratory trials.

Top 3 Questions About ISO 27001 Certification
Why is ISO 27001 certification important? What assurance does it provide? What do sponsors and CRO partners gain from working with an ISO 27001 certified company? Learn the answers to these questions in this info sheet.

When Has a Clinical Trial Ever Followed a Script?
MERIT’s customer service approach is unique. Find out how we drive study milestone achievement and increase site engagement.

Why Do You Need a Clinical Endpoint Expert? Top 4 Reasons
Any sponsor or CRO managing a drug development program that requires imaging endpoints knows the challenges that can arise. To address these challenges, you need a clinical endpoint expert. Here are four reasons why.

Do You Feel Like You Have Only One Choice for Your Respiratory Services Provider?
MERIT represents a reliable alternative in respiratory service providers, offering agile and attentive centralized spirometry expertise.

4 Main Challenges & Solutions for Spirometry in Clinical Trials
The right centralized spirometry partner can support your team and help you find efficient, reliable solutions for respiratory trials.

5 Common Imaging Study Challenges & How to Solve Them
Keep an eye on these 5 common imaging study challenges as you plan your future trials.

Decentralized Clinical Trial Technology Partner Checklist
Download our checklist for reference when selecting a DCT technology partner.

