MERIT provides consistent, high-quality independent reading center services to our CRO, pharmaceutical, and biotech partners.

Reading Center
Services
With MERIT’s Reading Center Services,
you benefit from the following:
- Standardized and accurate processes
- Reduced image acquisition variability among imaging sites
- Minimized bias in endpoint evaluation
- Increased data integrity in collection and interpretation
- Collaborative, partnership-driven approach
MERIT offers comprehensive Reading Center Services including:
Medical Expertise
- Study Design Insights
- Masked Image Read & Adjudication
Clinical Operations
- Development of Study-Specific Documents
- Site Technician and Equipment Certification
- Project Management and Site Support
- Global Reach
Technology
- Comprehensive Cloud-based Software Platform
- Image upload, evaluation, and QC
Quality Assurance
- Regulatory Compliance
- Data Privacy & Security
MEDICAL EXPERTISE
Study Design Insights
- Protocol design and consultation
- Reading criteria expertise
- Integration of published reading criteria with protocol and study needs
- Early involvement for protocol/imaging design/clinical data collection
- Advice on clinical endpoints and surrogate endpoints
Masked Image Read & Adjudication
- Uniform image evaluations across clinical sites by masked, independent readers
- Minimize inter/intra-reader variability through rigorous reader training, monitoring and oversight.
- All readers are trained for study-specific reading techniques according to the configuration of the protocol and the expectation of the sponsor.
CLINICAL OPERATIONS
Development of Study-Specific Documents
- Imaging charters
- Protocol and response-criterion driven, guide all imaging-related activities
- Site imaging manuals
- Provide instructions for sites on imaging schedule, image acquisition & upload, answering queries, site support contact info
- Reading criteria
- Modified and integrated based on protocol need
- Data management documents
- Edit checks, Data Transfer Specification, CDISC standardization
Site Technician and Equipment Certification
- Detailed user manuals and image acquisition procedures per the protocol
- Investigator Meeting, in-person or remote
- Web 1-1 training and instructions to site technicians
- Site (technician and equipment) certification to ensure readiness for FPI
- Supplemental performance information of a potential site based on prior experience
Project and Site Management
- Risk management through anticipating study challenges
- Data quality checks to ensure compliance
- Support ticketing system with <24hr resolution TAT
- Performance reporting
- Regular sponsor/site communications
Global Reach
- Images are collected from different sites around the world, standardized, and stored centrally
- EXCELSIOR delivers seamless image data sharing and evaluation
- Management of sites with different language requirements
- Global regulatory and data privacy compliance
TECHNOLOGY PLATFORM
Comprehensive Cloud-based Software Platform EXCELSIORTM
- One validated, secured system that contains all study data: images, tasks, queries, site materials, analysis, and read data
- Cloud-based platform bypasses site IT challenges and effectively manages access rights for sites, sponsors, and readers (zero footprint implementation)
- Eliminates the question: “Where is our data?” and reduces risk of data delays
- EXCELSIOR is a 510(k) cleared medical device for ophthalmic and radiological indications that is HIPAA compliant and meets all 21 CFR Part 11 requirements for electronic records and signatures
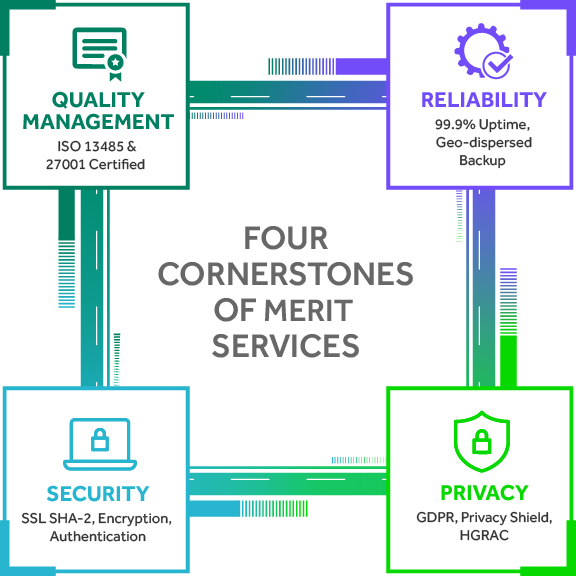
QUALITY ASSURANCE
MERIT’s commitment to quality is the foundation of our safe, reliable, and efficient data analysis and management services for clinical trials:
- Quality Management
- Reliability
- Security
- Data Privacy

MERIT Reading Center Services Solutions Guide

