Respiratory Disease States

Asthma is a chronic disease affecting adults and children in which the airways in the lungs narrow and swell, causing labored breathing, wheezing, chest tightness, and cough. According to the World Health Organization, asthma affected an estimated 262 million people in 2019 and caused 461,000 deaths. Asthma is the leading chronic disease in children.
Asthma cannot be cured, but medications and careful management of triggers can enable people with asthma to lead healthy, normal lives.
Whether studying a novel asthma treatment or performing a generic bioequivalence study, most asthma clinical trials include spirometry as a primary endpoint.
BRONCHODILATION (SERIAL SPIROMETRY): Serial spirometry is frequently used to assess a primary endpoint in asthma clinical trials for new or generic treatments that include Long-Acting Beta Agonists (LABAs), for example, to assess the drug’s action over a period of 12 hours. Bronchodilation is also used for a primary endpoint for studies assessing the change in forced expiratory volume in 1 second (FEV1) over time. For example, mean change in morning pre-dose FEV1 at time of randomization compared to the end of a 4-week treatment period.
BRONCHOPROVOCATION (METHACHOLINE, MANNITOL): Bronchoprovocation tests are sometimes used to diagnose asthma but are also used in clinical trials to establish bioequivalence. For example, methacholine bronchoprovocation challenges (MBPCs) are often the primary endpoint for generic albuterol sulfate studies. MBPCs are a dose-response test where subjects inhale an increasing dose of methacholine chloride solution via nebulizer, performing spirometry within a certain time frame after each dose, until they reach the provocative dose or provocative concentration of methacholine required to cause a 20% drop (PD20 or PC20) in FEV1, which is considered a positive challenge.
BRONCHODILATOR RESPONSIVENESS TESTING (REVERSIBILITY): Reversibility is the gold standard for diagnosing asthma and is very frequently an inclusion criteria for asthma clinical trials. For example, subjects would be required to demonstrate reversibility of airway obstruction, meaning an increase in FEV1 of ≥12% and ≥200 mL within 30 minutes of inhaling 360mcg of albuterol. This responsiveness to a bronchodilator such as albuterol strongly indicates the presence of asthma.
FRACTIONAL EXHALED NITRIC OXIDE (FeNO) MONITORING: FeNO is a simple, noninvasive method of measuring airway inflammation, more specifically T2, or allergic/eosinophilic inflammation. FeNO is measured by breathing into a portable device that assesses the level of nitric oxide in the air that is expelled from the lungs. FeNO could be used to predict the likelihood of responsiveness to inhaled corticosteroids (ICS) for population enrichment in clinical trials; for example, inclusion criteria in asthma trials to find responders to ICS.
PEAK EXPIRATORY FLOW (PEF): PEF (also sometimes referred to as peak expiratory flow rate, PEFR) is a person’s maximum speed of expiration. It is typically measured with a peak flow meter, which can be a manual, hand-held device, or an electronic device, often integrated with an electronic diary. PEF measurements provide an assessment of the degree of obstruction in the airways and are typically used in asthma clinical trials as a safety endpoint. Subjects will perform the measurements at home over the course of the trial. When PEF rates drop, it is often an indication of greater airway constriction.
COPD

Chronic Obstructive Pulmonary Disease (COPD) is a group of progressive lung disorders characterized by increasing breathlessness including emphysema and chronic bronchitis. It is a chronic inflammatory condition that causes obstructed airflow from the lungs. Symptoms include difficulty breathing, coughing, increased mucus production, and wheezing.
According to the World Health Organization, COPD is the third leading cause of death world-wide, causing 3.23 million deaths in 2019. Although there is no cure for COPD, it can be treated and managed with medications.
Whether studying a novel COPD treatment or performing a generic bioequivalence study, most COPD clinical trials include spirometry as a primary endpoint.
BRONCHODILATION (SERIAL SPIROMETRY): Serial spirometry is frequently used to assess a primary endpoint in COPD clinical trials for new or generic treatments such as long-acting muscarinic antagonists (LAMAs). The drug’s action on FEV1 over a period of 12 or 24 hours will be compared prior to and after treatment. For example, change from baseline in trough FEV1 on last day of treatment vs. a comparator.
BRONCHODILATOR RESPONSIVENESS TESTING (REVERSIBILITY): Reversibility is very frequently an inclusion criteria for COPD clinical trials as it is an important part of establishing a diagnosis of COPD. For example, subjects would be required to demonstrate a post-ipratropium FEV1/FVC ratio <0.7. A study participant might also need to show a post-ipratropium FEV1 < 50% of predicted normal and absolute FEV1 >700 mL.
PEAK EXPIRATORY FLOW (PEF): PEF (also sometimes referred to as peak expiratory flow rate, PEFR) is a person’s maximum speed of expiration. It is typically measured with a peak flow meter, which can be a manual, hand-held device, or an electronic device, often integrated with an electronic diary. PEF measurements provide an assessment of the degree of obstruction in the airways and are typically used in COPD clinical trials as a safety endpoint. Subjects will perform the measurements at home over the course of the trial. When PEF rates drop, it is often an indication of potential worsening of the disease.
ALLERGIC RHINITIS

Allergic rhinitis (AR) is an allergic response to environmental allergens such as pollens, weeds, dust mites, pet dander, insects, and molds that causes sneezing, nasal congestion, itchy nose, and sore throat. Allergic rhinitis can be seasonal in nature (to outdoor allergens) or perennial (indoor allergens).
According to the Centers for Disease Control and Prevention’s Summary Health Statistics for 2018, the number of adults diagnosed with Hay Fever (a common name for AR) in the previous 12 months was 19.2 million.
Managing seasonal or perennial AR can be accomplished by avoiding the allergens that trigger the reaction and by use of medications such as intranasal corticosteroids, antihistamines, and decongestants.
Clinical trials for treatments for AR usually include endpoints such as instantaneous and reflective total nasal symptom scores for rhinorrhea, nasal congestion, nasal itching, and sneezing. Other secondary endpoints might include assessment of ocular symptoms or quality of life measures such as the Rhinitis Quality of Life Questionnaire.
EIB

Exercise-Induced Bronchoconstriction (EIB) is a temporary narrowing and inflammation of the airways of the lung. Symptoms develop when the airways narrow due to physical activity and include shortness of breath, wheezing, coughing, and chest tightness. As many as 90% of people with asthma also have EIB, but not all people with EIB have asthma.
The prevalence of EIB in the general population is thought to be about 5-20%, but because few studies of EIB differentiate people with asthma from the general population, the true prevalence is hard to estimate.
EXERCISE CHALLENGE: Exercise challenges are a form of bronchoprovocation that can be used to diagnose asthma. It also is used as a primary endpoint in clinical trials for EIB. For example, it could be used to evaluate the protective effect of a once-daily treatment compared with a twice-daily treatment against EIB. The primary endpoint could be maximal percent decrease from pre-exercise FEV1 following exercise challenge at 12 hours post evening dose at the end of a 2-week treatment period.
CYSTIC FIBROSIS

Cystic Fibrosis (CF) is an inherited life-threatening disease caused by a defective gene that affects cells that produce mucus, digestive juices, and sweat. It is a progressive disease that damages the lungs, pancreas, and other organs. The mutation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene causes the CFTR protein to become dysfunctional. Without the CFTR protein functioning properly, chloride is unable to attract water to the cell surface, therefore leaving organs such as the lungs and the pancreas with thick and sticky mucus. The thick and sticky mucus congests the lungs airways obstructing airflow and blocks the release of enzymes in the pancreas designed to help digest food. Over time, this leads to decreased lung function, difficulty breathing and regulating mucus removal from the lungs, and malnutrition.
There are nearly 40,000 people in the United States living with CF, and it’s been estimated that over 100,000 people have been diagnosed with CF worldwide.
CFTR modulators are small molecules that treat CF by either correcting protein misfolding and misprocessing or improving channel gating. They enhance an array of clinical outcomes in CF from improved lung function, to increased BMI, to better quality of life outcomes.
However, for a minority of CF patients with rare and nonsense mutations, CFTR modulators are not effective, and preclinical and clinical research is ongoing for treatments to address this unmet need. Clinical trials for treatments for CF typically include endpoints such as change in FEV1, change in FEV1 % Predicted, and change in quality-of-life measures such as the Cystic Fibrosis Questionnaire-Revised (CFQ-R).
IDIOPATHIC PULMONARY FIBROSIS
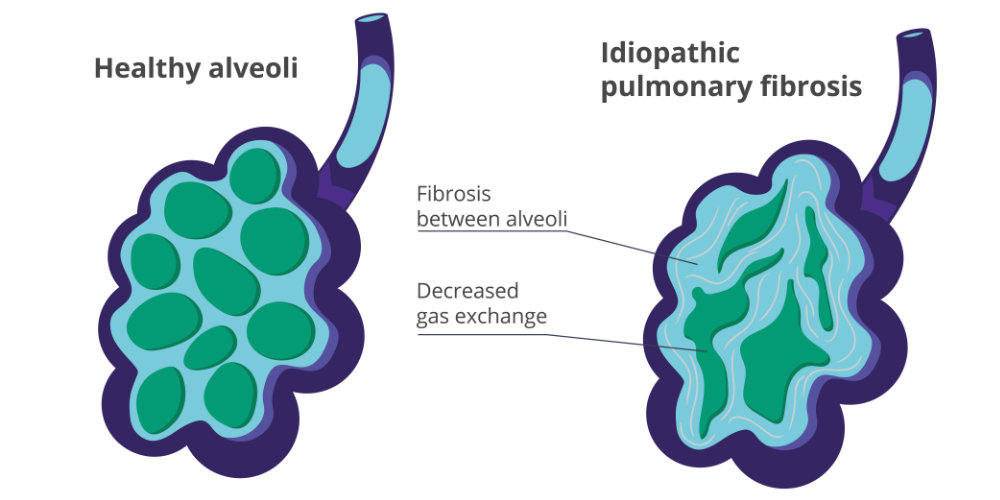
Idiopathic Pulmonary Fibrosis (IPF) is a progressive and irreversible lung disease characterized by the development of scar tissue (fibrosis) in the lungs. The term “idiopathic” means that the cause of the condition is unknown. In other words, IPF is a type of pulmonary fibrosis for which the underlying cause cannot be identified. IPF is categorized as a restrictive lung disease due to the decrease in lung elasticity. Scarring of the lung tissue makes the lungs become stiff, therefore expanding less, and decreasing overall lung function and oxygen update.
The fibrosis in the lungs makes it difficult for the lungs to expand and contract properly. This leads to a decrease in the amount of oxygen that can be transferred from the lungs to the bloodstream. Over time, as the scarring worsens, it becomes increasingly challenging for individuals with IPF to breathe, resulting in symptoms such as persistent cough, shortness of breath (especially during physical activity), and fatigue.
According to the American Lung Association, IPF is considered a rare disease but is more common than once thought, with up to 207,000 people affected in the United States and about 58,000 new cases diagnosed each year.
There is currently no cure for IPF, but clinical research is ongoing to find new and more effective treatment options. Clinical trials for IPF treatments include endpoints such as change in FVC, FVC percent predicted, and DLCO from baseline through the end of treatment period. Additionally, cough scores, breathlessness measures, and health-related quality of life endpoints might be examined.

