Key Takeaways
- ETDRS BCVA is the primary endpoint for vision improvement in many, if not most, ophthalmic clinical trials. Standardized measurement and collection of BCVA is key for successful trials.
- To measure clinically meaningful effects in persons with rare retinal disorders causing severe low vision, the FDA encouraged sponsors to create novel endpoints such as the MLMT
- Another example of a standardized measure developed to assess vision in severely affected patients with low vision and nystagmus is FST
BACKGROUND
In 2008, the Food and Drug Administration and the National Institutes of Health’s National Eye Institute sponsored an open symposium, the Ophthalmic Clinical Trial Design and Endpoints Symposium, that discussed developing standards for clinical trials in ophthalmology with particular focus on clinical endpoints used in vision research. One of the discussion sections concentrated on visual acuity parameters as outcome measures. The FDA views multiple measures of visual function as acceptable primary endpoints for clinical trials that evaluate the efficacy and safety of ophthalmic drugs including, but not limited to: visual acuity, visual fields, contrast sensitivity, and color vision.1 This section of the symposium also discussed that, while the Snellen Eye Chart had been an effective tool for clinical assessment of visual acuity for over 100 years, the Early Treatment Diabetic Retinopathy Study (ETDRS) Best Corrected Visual Acuity (BCVA) protocol offers greater standardization of visual acuity measurement in clinical trials and a “calculated visual acuity score” that can be used as a continuous variable for statistical analysis purposes.2 Areas of clinical research in ophthalmology have focused on the severely vision impaired, particularly in the area of gene therapy for blinding inherited retinal diseases (IRDs). As research has expanded in recent years, so too have clinical endpoints evolved over time.
VISUAL ACUITY TESTING: BAILEY-LOVIE AND ETDRS BCVA
ETDRS BCVA is the primary endpoint for vision improvement in many, if not most, ophthalmic clinical trials. Standardized measurement and collection of BCVA is key for successful trials. Standardization through certification and centralized data review supports accurate quantification of vision and consistent measures of vision change.
In 2013, Ian Bailey and Jan Lovie-Kitchin published their seminal article on the Bailey-Lovie visual acuity eye chart, citing the need for precision in visual acuity testing for low vision research as the basis of its design.3 They pointed out the importance of standardization in testing for clinical research, including having the same number of optotypes in each row, a constant ratio of size progression, and the spacing between optotypes within rows and between rows being proportional to the optotype size. Another key factor for standardization was the recording of visual acuity data in terms of the logarithm of the Minimum Angle of Resolution or logMAR. The logMAR method also allows for scoring letter by letter which provides greater precision in the measure of acuity.4
The ETDRS visual acuity chart is the best known “logMAR chart” that is based on the design principles of the Bailey-Lovie chart and has become the gold standard for BCVA testing. ETDRS BCVA is used for most research studies that have visual acuity as a primary endpoint. Like the Bailey-Lovie chart, the ETDRS chart has 5 letters at each size, size progression at exactly 0.10 log units, and standardized spacing between letters and rows. ETDRS also specifies acuity in LogMAR and gives equal credit for each extra letter read accurately. The differences between the two charts are the standard testing distance (6 meters for Bailey-Lovie versus 4 meters for ETDRS) and the letter families (1968 British Standard letters versus Sloan letters).5
BCVA AND BEYOND: VISUAL FUNCTION TESTING FOR LOW VISION RESEARCH
ETDRS BCVA has long been the method of choice for acuity testing in clinical trials, but BCVA is just one of the pillars of measuring visual function. Important visual function outcomes also include contrast sensitivity, color, depth, and motion as well as visual fields.6 As Bailey and Lovie noted even back in 2013, new developments attempting to restore or enhance vision in blind or severely visually impaired persons would require new clinical tests.7
Multi-Luminance Mobility Testing
In its 2020 guidance document, “Human Gene Therapy for Retinal Disorders,” the Food and Drug Administration (FDA) recognized this need for new endpoints. They encouraged sponsors to create and suggest novel endpoints to measure clinically meaningful effects in persons with retinal disorders, particularly with rare retinal disorders where traditional efficacy endpoints may not be appropriate. The FDA cited the example of a new primary efficacy endpoint measuring mobility under different levels of illumination, which was used to help establish marketing approval for Spark Therapeutic’s LUXTURNA (voretigene neparvovec-rzyl).8
The novel endpoint referred in the FDA guidance, known as Multi-Luminance Mobility Testing (MLMT), was first developed for a Phase I trial of gene therapy (LUXTURNA) for RPE65-mediated IRDs such as Leber Congenital Amaurosis (LCA). Untreated RPE65-mediated IRDs eventually cause patients to lose the ability to detect light of any intensity, making independent navigation severely limited.9 The MLMT combines aspects of visual acuity, visual field, and light sensitivity into a quantifiable measure. For this test, subjects are instructed to follow arrows on the MLMT course while avoiding obstacles on or near their path, navigating raised steps and detecting a door at the end of the course. The course can be conducted at up to nine standardized light levels, with one eye patched and/or both eyes unpatched. Each course attempt is video and audio recorded and is typically graded by independent, masked graders.10 The MLMT was used in further Phase I and Phase III studies, and validated in another study as being able to distinguish normal-sighted from visually impaired subjects. In addition, it could identify a range of performance in visually impaired subjects, as well as tracking performance declines over time in this population.11
The MLMT assesses an individual’s functional vision, or ability to conduct visually dependent activities of daily living independently, which has great impact on quality of life. Perhaps just as importantly, it offers a standardized and quantitative assessment for use in clinical trials.
Full-Field Stimulus Testing
Another example of a standardized measure developed to assess vision in severely affected patients with low vision and nystagmus is Full-field Stimulus Testing (FST). The original purpose for FST was as a technique to quantify visual thresholds in LCA, where traditional outcome measures, such as visual acuity and visual fields, are difficult if not impossible for most patients to perform. The FST session estimates the threshold, or stimulus intensity, that is barely detectable by the patient. Stimuli of different intensities are presented, and the patient indicates if it is seen or not by pressing a response button. To estimate the threshold efficiently, an algorithm is used to adaptively choose the intensities presented.12
While FST was developed originally as a method to assess the severe vision impairment of LCA patients, its accessible and convenient means of measuring dark-adapted sensitivity with chromatic stimuli has also seen use in the study of other rare IRDs such as Stargardt disease and Late-Onset Retinal Degeneration (L-ORD).13 FST may also be paired with MLMT because the ceiling effect inherent in MLMT can partially limit assessment of improvement in functional vision assessment for RPE65-LCA patients. Pairing the two extends the dynamic range of the MLMT. In addition, the visual deficit in other genotypes of LCA may not be as prominent for rods as for cones, and therefore would be better assessed using FST rather than mobility courses.14
SUMMARY
Developing standards for clinical endpoints used in vision research is clearly of paramount importance in discovering new treatments for ophthalmic diseases. ETDRS BCVA has been a foundational endpoint, but in recent years, areas of clinical research have extended to the severely vision impaired, particularly in gene therapy for blinding IRDs. As research has expanded in recent years, so too have clinical endpoints evolved over time.
MERIT Services
MERIT is a specialty ophthalmology core lab supporting Phase I – IV clinical trials. Our team are experts in managing and evaluating images, especially with ocular diseases affecting the retina. As a trusted partner to sponsors and CROs, we provide domain expertise, innovative imaging tools, and scalable solutions.
MERIT offers comprehensive training, certification, and management of study Best Corrected Visual Acuity (BCVA) activities as well as centralized data review of BCVA source documents. Services include:
- Full, Abbreviated, and Express Certifications for VA Examiners
- Full and Express Certifications for VA Rooms
- Certificates Maintained in EXCELSIORTM
- Confirmation of Acceptable BCVA Equipment
- Scheduling, Tracking & Reporting of BCVA Certification Study Activities
- Visual Acuity Certifications: ETDRS, Low Luminance, Contrast Sensitivity & Reading Assessments
- VA Room Equipment Procurement
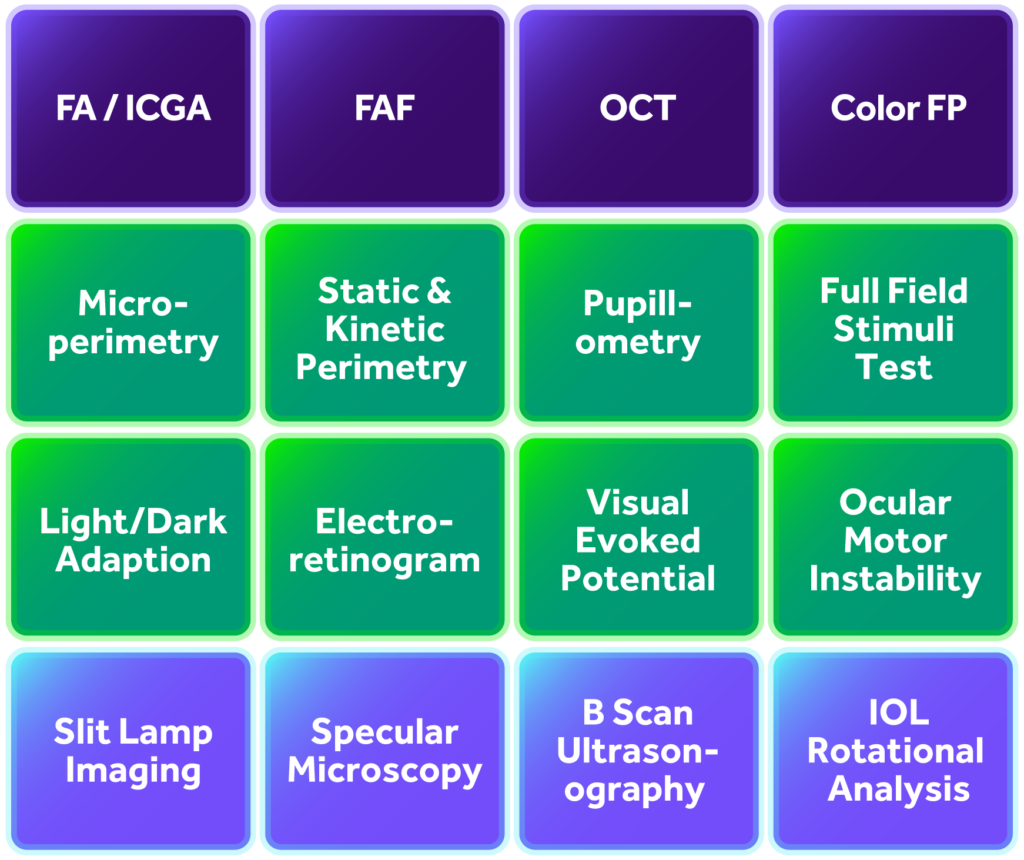
MERIT’s imaging services also support the following modalities:
References
1Csaky KG, Richman EA, Ferris FL 3rd. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008 Feb;49(2):479-89. doi: 10.1167/iovs.07-1132. PMID: 18234989.
2Ibid., p. 480.
3Bailey IL, Lovie-Kitchin JE. Visual acuity testing. From the laboratory to the clinic. Vision Res. 2013 Sep 20;90:2-9. doi: 10.1016/j.visres.2013.05.004. Epub 2013 May 17. PMID: 23685164.
4Ibid., p. 3-4.
5Ibid., p. 4.
6Bennett CR, Bex PJ, Bauer CM, Merabet LB. The Assessment of Visual Function and Functional Vision. Semin Pediatr Neurol. 2019 Oct;31:30-40. doi: 10.1016/j.spen.2019.05.006. Epub 2019 May 11. PMID: 31548022; PMCID: PMC6761988.
7Bailey, Lovie-Kitchin, p. 7.
8US Department of Health and Human Services, Food and Drug Administration. Human Gene Therapy for Retinal Disorders: Guidance for Industry. January 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-gene-therapy-retinal-disorders
9Chung DC, McCague S, Yu ZF, Thill S, DiStefano-Pappas J, Bennett J, Cross D, Marshall K, Wellman J, High KA. Novel mobility test to assess functional vision in patients with inherited retinal dystrophies. Clin Exp Ophthalmol. 2018 Apr;46(3):247-259. doi: 10.1111/ceo.13022. Epub 2017 Aug 31. PMID: 28697537; PMCID: PMC5764825.
10Ibid., p. 249.
11Ibid., p. 247.
12Roman AJ, Cideciyan AV, Wu V, Garafalo AV, Jacobson SG. Full-field stimulus testing: Role in the clinic and as an outcome measure in clinical trials of severe childhood retinal disease. Prog Retin Eye Res. 2022 Mar;87:101000. doi: 10.1016/j.preteyeres.2021.101000. Epub 2021 Aug 28. PMID: 34464742.
13Ibid., p. 9.
14Ibid., p. 11.