MERIT Webinar Summary
Most brain cancer diagnoses in the United States are glioma, and MERIT’s webinar explored the challenges and potential solutions for assessing these tumors. These primary brain tumors can present in the form of gliomas (both low-grade glioma and glioblastoma). Oftentimes glioma can be debilitating to patients with a poor prognosis and limited treatment. New therapies are being studied through clinical trials, many of which use response criteria such as Response Assessment in Neuro-Oncology (RANO). Pseudoprogression (PsP) occurs when enhancement on imaging may be interpreted as progression but is actually an effect of the treatment and is not considered true progression. PsP has proven a challenge for many researchers and radiologists.
In our webinar, Dr. Chad Farris, MD, PhD, and Dr. Gene Kim, PharmD, shared thoughts on assessing PsP and reader challenges, and discussed how to incorporate accurate outcomes and collect high-quality data in clinical trials. This article summarizes the portion of the webinar presented by Dr. Farris.
To view the entire webinar entitled “Navigating Neuro-Oncology: Understanding Pseudoprogression and Harnessing PsP Outcomes for Optimal Imaging Assessment,” click here.
Introduction
By way of introduction, Dr. Farris provided some background on the epidemiology of brain tumors. About one-third of brain tumors are malignant, and of those, around 80% are gliomas. More than 50% of gliomas are glioblastomas, which are the worst of the malignant brain tumors in terms of survival rate:
- 1-year survival 37.4%
- 5-year survival 4.9%1
Dr. Farris explained that gliomas are tumors of the supporting cells (glial cells) of the central nervous system. There are three adult-type diffuse gliomas according to the 5th Edition of WHO Classification of Tumors of the Central Nervous System:
- Astrocytoma, IDH-mutant (grade 2, 3, or 4)
- Oligodendroglioma, IDH-mutant, and 1p/19q-codeleted (grade 2 or 3)
- Glioblastoma, IDH-wildtype (grade 4)2
Gliomas are divided into low-grade versus high-grade. Low-Grade Glioma (LGG) refers to Grade 1 and 2 gliomas, while High-Grade Gliomas (HGG) refers to Grade 3 and 4 gliomas.
Finally, Dr. Farris described the typical treatment approach for glioblastoma. Step 1 is surgical resection of the greatest amount of enhancing disease possible (the amount of residual enhancing disease is an independent predictor of survival). Step 2 is radiotherapy plus concomitant and adjuvant Temozolomide.34
Having established that glioblastoma is the most common form of malignant brain tumor that also has a very poor prognosis despite the best possible treatment, it’s clear why new treatments for glioblastoma are critical. Assessing response to new therapies in clinical trials is also important.
Criteria for Assessing Response to Therapy
Dr. Farris next outlined the timeline for the introduction of criteria for assessing response to therapy, along with a brief overview of each of the response assessment criteria. Dr. Farris began with early response criteria that had no, or very early, descriptions of PsP.
- 1977 – Levin Criteria (J Neurosurg. 1977 Sep;47(3):329-35.) These criteria were the first to attempt to differentiate progression from response and came out when CT technology was first being introduced. The criteria utilized neurologic exam, radionuclide scans, very early CT scans, and EEG. There was no discussion of PsP
- 1990 – Macdonald Criteria (J Clin Oncol. 1990 Jul;8(7):1277-80.) The next criteria developed was Macdonald. This was the first criteria that laid out the categories of response as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). It utilized contrast-enhanced CT or MR along with neurologic exam. PsP is mentioned, but they discussed it as neurological decline that wasn’t due to tumor
Dr Farris also gave a brief overview of the RANO criteria as it evolved from 2010 to 2023, all of which deal with PsP, which will be discussed in detail below.
- 2010 – RANO (Response Assessment for Neuro-Oncology) Criteria (J Clin Oncol. 2010 Apr 10;28(11):1963-72.) The original RANO dealt with HGG only and was the first response criteria that discussed PsP in the same way it is understood today
- 2011 – RANO Criteria for LGG (Lancet Oncol. 2011 Jun;12(6):583-93.) Primary distinction for this criteria is that HGG usually enhance while LGG don’t usually enhance and are therefore examined with T2/Flair imaging
- 2015 – iRANO (immunotherapy) (Lancet Oncol. 2015 Nov;16(15):e534-e542.) Provided distinct criteria for immunotherapy treatments
- 2017 – mRANO (modified) (Neurotherapeutics. 2017 Apr;14(2):307-320.) Dealt with HGG and were an attempt to update the criteria with the best available research.
- 2023 – RANO 2.0 (J Clin Oncol. 2023 Sep 29:JCO2301059. doi: 10.1200/JCO.23.01059. Online ahead of print.) The most recent response criteria, also reflecting the most recent research
What is Pseudoprogression?
PsP was first defined in the RANO Criteria in 2010 as increased contrast enhancement on a post-radiation scan that eventually improves without a change in therapy. In other words, it looks like it was tumor, but when you follow it without changing the therapy, it doesn’t get worse, indicating that it wasn’t truly PD but was PsP.
At the time of RANO in 2010, it was believed PsP occurred in 20-30% of patients on the first post-radiation scan. Currently for patients with glioblastoma, it’s even higher, probably in the neighborhood of 30 to 40%. PsP is thought to be due to increased permeability of the tumor vasculature from irradiation, which may be enhanced by temozolomide. Typical therapy is the combination of irradiation with concomitant and/or adjuvant temozolomide for glioblastomas, and it’s thought that both factors may contribute to PsP.
PsP can be accompanied by clinical signs and symptoms, but usually it’s not, whereas true PD tends to always be eventually associated with clinical signs and symptoms. PsP is more frequent in patients with a methylated MGMT gene promoter.5 This is important because such patients are more likely to have a better response to therapy, so PsP is more prevalent in the people who actually may have a better response.
Here are two examples of PsP cases from RANO 2010.
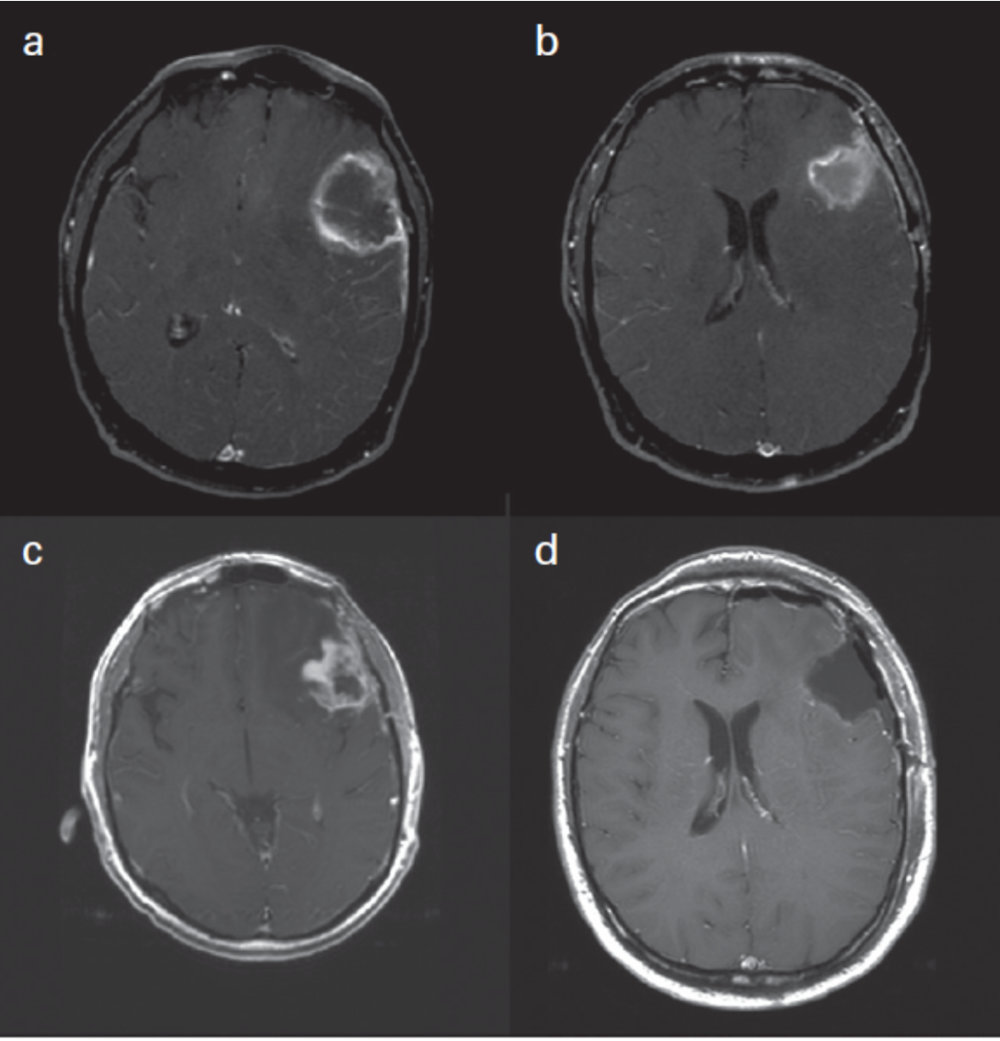
Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010 Apr 10;28(11):1963-72, p.1965
Panel A shows a peripherally irregular enhancing mass with central necrosis or non-enhancement. Panel B shows the post-resection MRI showing the resection cavity. Panel C shows the follow-up MRI with thick irregular enhancement around the periphery of the resection cavity which was worrisome for disease progression. Panel D shows the post re-resection MRI where all enhancing disease has been removed. However, no viable tumor was recovered; it was all necrosis. Therefore, this is an example of surgically confirmed PsP.6
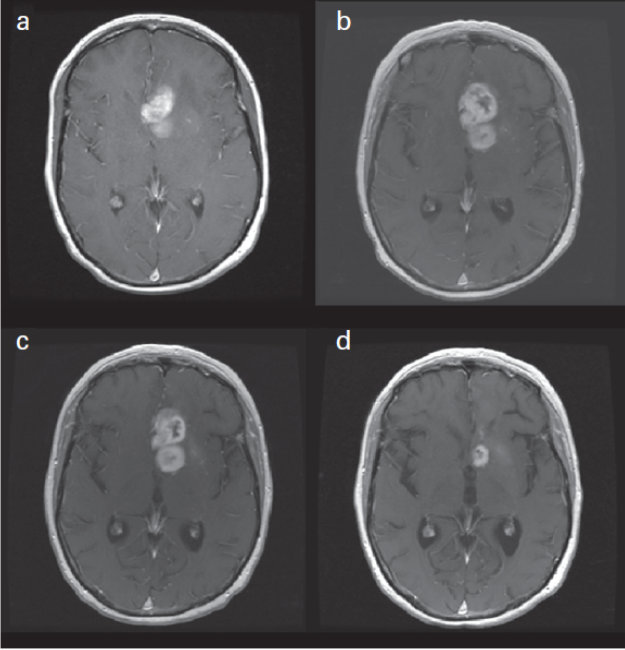
Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010 Apr 10;28(11):1963-72, p.1965
Panel A shows a scan two-days after biopsy proven glioblastoma was diagnosed. Panel B shows the post-radiation scan which shows enlargement of the area of abnormal enhancement looking like disease progression with a concern for PsP. Panel C shows the follow-up MRI 4 weeks later with no evidence of change and no change in therapy. Panel D shows the scan after the completion of 8 cycles of temozolomide which shows the tumor, or what was thought to be a tumor, has improved so that the area of enhancement has significantly improved with no change in therapy. Thus, this is a strong example of PsP.7
The following is another PSP example from the iRANO Criteria of 2015.
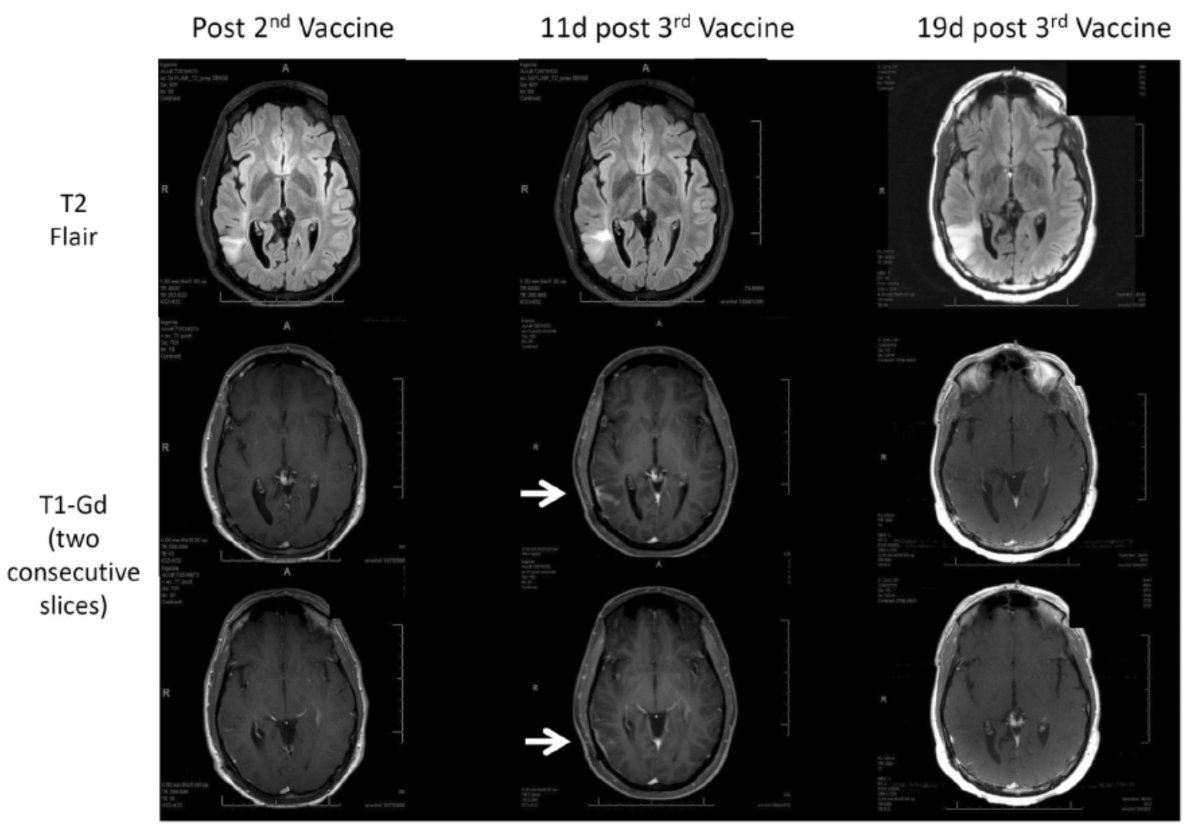
Immunotherapy Response Assessment in Neuro-Oncology (iRANO): A Report of the RANO Working Group. Lancet Oncol. 205 Nov;16(15):e534-e542
This is oligodendroglioma being treated with a vaccine therapy. The first column shows the scans after the 2nd vaccine with areas of T2 flair hyperintensity corresponding to the tumor. The two T1-Gd scans below it show no abnormal enhancement. After the 3rd vaccine, they did develop abnormal enhancement (white arrows) on the scan 11 days after vaccine. However, 19 days after the 3rd vaccine, the abnormal enhancement had resolved, so this is another example of PsP.8
What Problems Arise with Pseudoprogression?
There are three major problems with PsP as outlined in RANO 2010. The first is that it may lead to premature discontinuation of effective adjuvant therapy. For example, let’s say the patient has undergone surgery and is on temozolomide, but a follow-up scan seems to show progression of disease. If that progression turns out to be PsP, the temozolomide is stopped even though it’s having a beneficial effect. Thus, for an individual patient you have a negative consequence of stopping the therapy that’s working because there was thought to be progression.
A second problem with PsP is that it limits the validity of Progression Free Survival as an end point unless tissue-based confirmation of tumor is obtained. This is because it seems the patient has progressed sooner than they did on a therapy, so a shorter interval of Progression Free Survival is assigned instead of what they really had.
The third problem that arises with PsP is the failure to exclude patients with PsP from clinical trials for recurrent glioma, which will give falsely high response rates and Progression-Free Survival. If it’s PsP and a patient stops routine therapy and goes on a clinical trial for recurrent glioma, the problem is that there hasn’t been a recurrence of the tumor. Then, when there’s stable disease or even a response on the treatment, it’s because the PsP enhancement has improved and it’s not actually a treatment response.
How Do Assessment Criteria Deal with Pseudoprogression?
RANO Criteria in 2010
With the original RANO criteria, progression can only be determined if the majority of the new abnormal enhancement is outside of the radiation field (beyond the high-dose region or 80% isodose line) within the first 12 weeks after completion of radiotherapy. If it’s outside the field, the abnormal enhancement is not being caused by the radiation exposure. The only way to declare progression within the first 12 weeks after radiotherapy when the new enhancement is within the radiation field, is to find unequivocal evidence of viable tumor on histopathological sampling.
If it’s 12 weeks after completion of radiotherapy or beyond, progression is found if:
- New contrast-enhancing lesion outside radiation field (same as within the first 12 weeks after radiation)
- Increase by > 25% in the sum of products of the diameters between the first postradiotherapy scan, or a subsequent scan with smaller tumor size, and scan at 12 weeks or later, on stable or increasing doses of steroids
- Clinical deterioration not attributable to other cause (can be considered progression – but not for entry into clinical trial)
- If getting antiangiogenic therapy, significant increase in T2/FLAIR may be considered for progression with stable or increasing steroids vs. baseline9
iRANO Criteria in 2015
The iRANO criteria made additions to the RANO 2010 criteria by incorporating clinical status and length of immunotherapy into determining progression. Essentially, under iRANO, if a scan shows radiologic progression, the question must be asked as to whether there is significant clinical deterioration. If there is significant deterioration in the presence of radiologic progression, that will be considered PD and the immunotherapy regimen will be discontinued.
If there is not clinical decline in the presence of radiologic progression, then the duration of the current immunotherapy regimen is considered. If the patient has been on the regimen for greater than 6 months, that will also be considered PD and the immunotherapy will be stopped. If the patient has been on the regimen for less than or equal to 6 months, then the therapy is continued for 3 months as long as there is no decline in clinical condition and the patient stays stable for those 3 months. There is a follow up MRI after 3 months that is used to determine if this is true PD or if it was PsP.
If radiological progression is found on the follow-up scan, then progression is declared. If radiologic progression is not found, a different response such as CR, PR, or SD is assessed and the immunotherapy is continued. If it is true PD, the date of progression is backdated to the initial finding of radiologic PD.
mRANO Criteria in 2017
One of the major modifications of mRANO was the use of the post-radiation time point as the baseline for response evaluation in newly diagnosed GBM vs. the pre-radiation scan that was previously the baseline. This was important because things can develop during that time, and this is a significant change in baseline evaluation as compared to the original RANO criteria.10
In addition, for mRANO, the definition of response and progression is based on only considering objectively defined, measurable enhancing disease and excluded qualitatively assessed T2/FLAIR changes. In other words, they stopped using the enlargement of the T2/Flair area for enhancing tumors as a marker of progression. Finally, mRANO requires two scans, 4-8 weeks apart, to prove progression.
The following is a simplified overview of the process of assessing PD and PsP in a clinical trial using mRANO:
- Begins with diagnosis and pre-entry MRI, followed by surgical resection and then a post-operative MRI (0)
- Patient goes on radiotherapy and treatment concurrently, after which they undergo the post-treatment MRI (1) which forms the baseline for response assessment. They will then start adjuvant therapy
- Post treatment, they undergo MRI (N). If there is a >25% increase in bidimensional measurement of enhancing disease or > 40% increase in volumetric measurement of enhancing disease at the MRI (N), preliminary PD is declared
- Patients remain on therapy for another 4-8 weeks at which point they have MRI (N+1), which is compared to the post-treatment baseline MRI(N), and if there is a >25% increase in bidimensional measurement or > 40% increase in volumetric measurement Confirmed PD is declared and therapy is stopped
- If SD, PR, or CR is found with MRI (N+1), Confirmed PsP is declared, and the subject continues therapy and has another MRI 4 to 8 weeks later, MRI (M)
- If >25% increase in bidimensional measurement or > 40% increase in volumetric measurement is found on MRI (M) in comparison to Nadir or MRI (N+1), Confirmed Progression is found and therapy stops. Date of progression is backdated to the post-treatment MRI(N)
- If SD, PR, or CR is found on MRI (M) SD is declared and the patient continues on therapy
The procedure for assessing PD is very similar for recurrent GBM, except that the initial part of the procedure is somewhat different. Recurrent GBM in mRANO:
- Begins with recurrence and pre-entry MRI (0)
- The subject may undergo re-resection of the enhancing disease, but this is optional
- New baseline pre-treatment MRI (1) occurs after the surgery (if performed) and must take place within 72 hours of surgery. Ideally it would be closer to surgery, but the criteria allows 72 hours
- Patient is randomized to treatment, and therapy must start within 21 days of the pre-treatment MRI (1)
- Following treatment, the procedure follows the same outline as for newly diagnosed GBM
Some additional key factors that were introduced with mRANO include:
- T1 subtraction map was suggested to best identify enhancing disease. The pre-contrast T1-weighted MRI is subtracted from the post-contrast T1-weighted MRI to get the T1 subtraction map which greatly highlights the enhancing disease
- mRANO also offered the option of lesion measurement by either the traditional biplane measurement or volume measurement. There must be > 40% increase in volumetric measurement of enhancing disease as opposed to > 25% increase in bidimensional measurement to declare PD
- mRANO criteria recommend 3D T1 pre- and post-contrast imaging in accordance with the International Standardized Brain Tumor Imaging Protocol. Prior to mRANO, 2D imaging was acceptable
RANO 2.0 Criteria in 2023
The RANO 2.0 criteria are the most recent response criteria and were published in September 2023.11 One of the first items discussed in RANO 2.0 is baseline scans. RANO 2012 stated the baseline scan should be the post-surgery MRI, but mRANO suggested the post-radiotherapy scan should be the baseline. RANO 2.0 recommends using the first post-radiotherapy scan at approximately 4 weeks after radiotherapy as the baseline to determine PD.
If the diagnosis is of a new glioma that is not getting radiotherapy, then the post-surgery scan should be the baseline and it should take place less than 48 hours after surgery. They shortened that interval from mRANO from 72 to 48 hours because the longer you wait after surgery, the greater the chance that you see enhancement that is not actually enhancing tumor but response to surgery. With recurrent glioma the pretreatment MRI will be the baseline as was the case with mRANO.
RANO 2.0 also recommends that the baseline scan be performed as close as possible to the initiation of therapy but not more than 14 days. This timeframe was 21 days in mRANO, so RANO 2.0 has shortened that window.
It’s worth noting that even though the post-surgery scan is no longer being used as the baseline, it is still useful to detect complications from surgery. In addition, you can assess the extent of resection of enhancing disease, which has prognostic value. The more enhancing disease that is left behind after surgery, the worse the prognosis is for the patient.
RANO 2.0 discusses the criteria for declaring PD to allow entry into trials for recurrent glioma. The criteria for declaring PD include:
- Greater than or equal to 25% increase in sum of products of perpendicular diameters of lesions (same as in original RANO)
- Or equivalently, greater than or equal to 40% increase in volume of enhancing lesions
- New measurable lesion
If imaging is concerning for progression in the first 12 weeks after chemoradiation and the patient is clinically stable, RANO 2.0 recommends the patient get follow-up at 4- or 8- week intervals to confirm PD and rule out PsP, which is seen in 30-40% of GBMs in first 12 weeks after radiation treatment. Note that if progression is confirmed on follow-up imaging it should be backdated to first evidence of PD and not the follow-up scan that confirmed PD. This would affect the time of Progression-Free Survival, so PD should be dated at the first signs of PD, even if a follow-up scan was needed to confirm.
Notably, with RANO 2.0, a second confirmation scan is not required to confirm PD if you’re not concerned about PsP outside the first 12 weeks, which is different from mRANO.
RANO 2.0 also outlines when patients should be excluded from trials for recurrent glioma. Patients should be excluded when progression is in the first 12 weeks after chemoradiation unless the progression is clearly outside the radiation field (beyond high-dose region or 80% isodose line) or if there is pathologic confirmation of disease progression. Note the limitations of the reliability of pathology in this time period and that pathologists have a hard time distinguishing PD and PsP during this window.
Finally, it should be noted that PsP for IDH-mutated gliomas (not GBMs) can extend well beyond 3 months from radiotherapy, so an optional confirmation second scan can be obtained even if you’re outside the 12-week window. For a GBM trial, however, if you’re outside the 12-week window, it is probably PD and a second scan is not required.
Advanced Imaging Methods to Differentiate Between True Progression and Pseudoprogression
There are several advanced imaging methods that have become common clinically but have not been part of the RANO criteria. RANO 2.0 states that advanced imaging techniques may help differentiate progression from PsP, but “require further validation before formal incorporation into RANO 2.0.” Examples of advanced imaging techniques that can help distinguish PD from PsP include (noting these are not included in RANO):
- Diffusion MRI
- Dynamic Susceptibility Contrast (DSC) (Perfusion) MRI
- Dynamic Contrast-Enhanced (DCE) (Perfusion) MRI
- Amino Acid Positron Emission Tomography (PET) Imaging
- Magnetic Resonance Spectroscopy (MRS)
The first illustration below is of MRS.
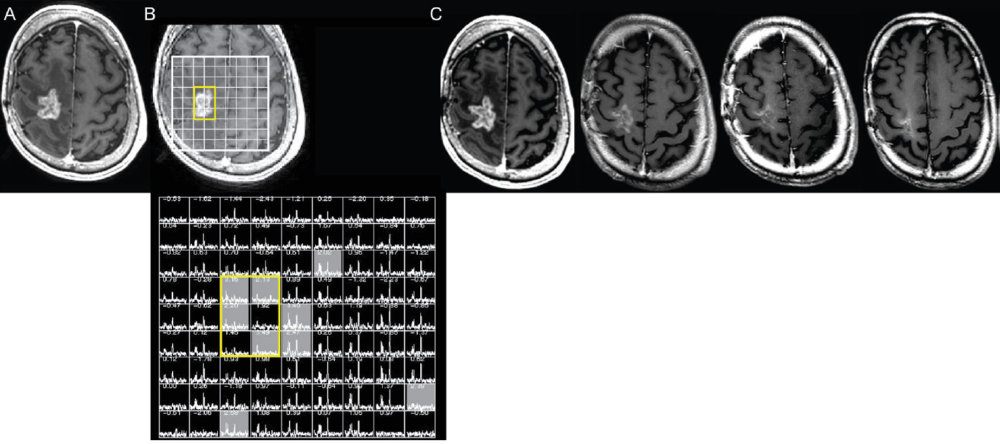
Pseudoprogression versus true progression in glioblastoma: what neurosurgeons need to know. J Neurosurg. 2023 September 01; 139(3): 748-759, p. 752
Panel A shows an enhancing mass on post-contrast imaging. Panel B shows overlaying a grid doing multi-voxel spectroscopy and the area in yellow is centered over the enhancing mass. The grid below Panel B shows there are NAA and choline peaks in the normal brain regions (outside the yellow box) but in the area of the enhancing mass there’s no NAA peak and no choline peak. Decreased NAA and decreased choline is consistent with PsP, because with tumors, you would expect an elevated choline peak and a reduced NAA peak. The follow-up scans shown in Panel C indicate a decrease in the area of enhancement over eight months, which is consistent with PsP as predicted by the MRS.12
The next example is DSC-Perfusion. Panel G shows another tumor that was irradiated showing a large area of T2 hyperintensity. Panel H shows the irregular enhancement around the area of central necrosis. On the perfusion map of relative cerebral blood volume in Panel I, however, there’s no area of elevated cerebral blood volume that corresponds with the areas of thick irregular enhancement shown in Panel H. This is suggestive of PsP which was confirmed with further surgical resection with no viable tumor detected.13
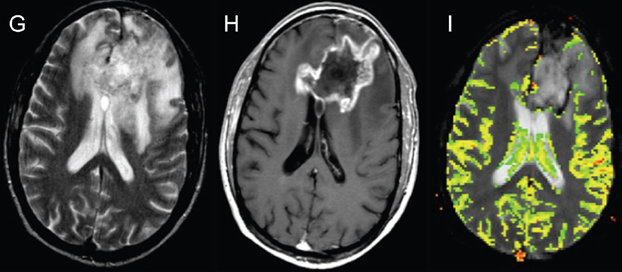
Pseudoprogression versus true progression in glioblastoma: what neurosurgeons need to know. J Neurosurg. 2023 September 01; 139(3): 748-759, p. 751
Highlights from the Q and A
Q: What do you think is the most significant change to the RANO criteria with the new version that was just released?
A: This is a great question. I would address it with two thoughts. One important factor is that RANO 2.0 is treatment-agnostic, which is new. Before this we had the mRANO which dealt with high-grade gliomas, then we also had the RANO-LGG which dealt with the low-grade glioma criteria, and we had iRANO to deal with immunotherapy treatments. Now RANO 2.0 is treatment-agnostic, so it’s for all types of therapies, and it also captures both HGG and LGG, all in one set of response criteria. Another important factor for GBM in particular is that if the scan is outside of the 12-week window, you no longer need to have a follow-up scan to confirm PD unless there is some strong reason to think otherwise. This is in contrast with mRANO where you were required to have two scans to show disease progression.
Q: Should necrotic region within T1 post-contrast lesions be taken into account and included when applying volumetric measurements for RANO 2.0?
A: This is an interesting question and it gets at one of the problems with using volumetric measurements. The necrotic region should not be included within the volumetric measurement, which can potentially pose a problem for such measurements. It’s important to only measure the enhancing disease in the volumetric measurements and exclude the portion of the lesion that is non-enhancing, and that can sometimes be problematic. I think is one of the reasons why the bi-plane measurements are still the predominant method which is being suggested in RANO 2.0, even though it gives the option of using volumetric measurements. I don’t think we have a great way of separating out the non-enhancing disease in some situations, but it is the case that it should not be included in the volumetric measurements.
Q: My company is developing a Phase II clinical trial, and we were using the published RANO guidance. With the new 2.0 version being released, should we consider making changes to our trial?
A: That’s an excellent question, too. It comes down to basically two points, depending on where you are in the clinical trial. If it’s still early enough that you haven’t started imaging, you can consider the new adjustments for RANO 2.0 for the baseline criteria for determining what’s used for the baseline scan. If the patient’s going to be getting radiation, you can use the scan that is obtained 4 weeks after radiation. If it’s a patient that is not going to get radiation for a new glioma, you would use the post-surgical MRI within 48 hours after resection as the baseline scan. For recurrent glioma it would be the pre-treatment scan which would need to be within 14 days of when the new treatment was started. This would all be possible if you hadn’t started the trial and were able to make these adjustments. If you have already started the trial, you could still think about the fact that you no longer need two scans to confirm progression. For example, if you’re looking at glioblastoma, and you’re looking at what seems to be progression outside the 12-week window after radiation therapy, no additional imaging is required to confirm PD. If what looks like PD shows up within the 12-week window after radiation, you still need additional imaging to confirm PD.
Why MERIT?
Innovative. Proven. Experienced.
MERIT has provided image collection, centralized reading, and data management services for clinical trials since 2012.
Using our innovative and proven technologies and intuitive, seamless workflows, MERIT’s experienced staff bring more than a decade of clinical endpoint expertise to ensure the success and integrity of your independent imaging review studies.
- Experience in managing clinical trials
- Single platform for all study data, queries, reporting/tracking, image transfer and analysis
- Oncology experienced staff
- Step-by-step Imaging Protocols, SOPs, and document support
- Indispensable regulatory support and guidance
- Established, time-tested Quality Assurance, security, and data privacy
If you’re looking for a partner that will bring dedication and expertise to your clinical trial, consider MERIT.
To watch the webinar “Navigating Neuro-Oncology: Understanding Pseudoprogression and Harnessing PsP Outcomes for Optimal Imaging Assessment” in its entirety, click here.
References
1Neurol Clin 36 (2018) 395–419
2Neuro Oncol. 2021 Aug; 23(8): 1231–1251.
3Neuro Oncol. 2018 Aug 2;20(9):1240-1250.
4N Engl J Med. 2005 Mar 10;352(10):987-96.
5J Clin Oncol. 2010 Apr 10;28(11):1963-72.
6Ibid.
7Ibid.
8Lancet Oncol. 2015 Nov;16(15):e534-e542.
9J Clin Oncol. 2010 Apr 10;28(11):1963-72.
10Neurotherapeutics. 2017 Apr;14(2):307-320.
11J Clin Oncol. 2023 Sep 29:JCO2301059. doi: 10.1200/JCO.23.01059. Online ahead of print.
12J Neurosurg. 2023 September 01; 139(3): 748–759.
13J Neurosurg. 2023 September 01; 139(3): 748–759.