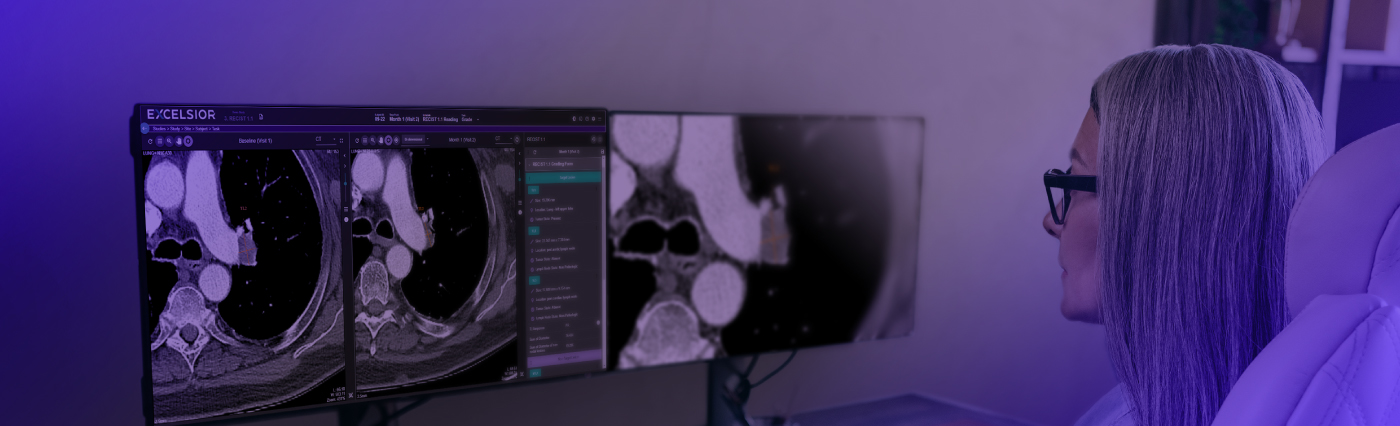
RECIST 1.1 and Oncology Trial Endpoints
RECIST 1.1 is the standard response criteria for solid tumors and has been a basis for adaptation and variation of other response criteria such as mRECIST, irRECIST, iRECIST, and so on. In this webinar, MERIT discusses the principles of RECIST 1.1, the information needed to collect imaging endpoints, and special considerations while using the RECIST 1.1 criteria.
3 key questions
that are answered:
- What key elements are collected into the eCRF form while reading RECIST 1.1?
- How do I derive imaging endpoints from RECIST 1.1?
- What are the common mistakes and special considerations during the data collection of RECIST 1.1?

Gene is a Clinical Scientist providing subject matter expertise and helping design and configure MERIT’s flexible oncology reading platform to accommodate various reading paradigms. He has experience in evidence-based patient care and academic research, a background in pharmacy, and 6 years supporting clinical trials. Gene implements software workflows to support oncology trials with response criteria such as RECIST 1.1, iRECIST, Lugano, and RANO, and helps to increase the efficiency and accuracy of clinical data collection for imaging endpoints. He received his Doctor of Pharmacy (Pharm.D.) from the University of Wisconsin-Madison and has a bachelor’s degree in Neurobiology.

Shinji Yue leads the oncology commercial strategies at MERIT. He has 20+ years of global commercialization and market development experience in medical imaging, AI software, and medical devices for clinical trials. Shinji is responsible for growing the oncology business; developing commercial relationships with sponsors; and building and managing an exceptionally talented team. He received his MBA in Marketing and Finance, MS in Microbiology, Immunology, and Cancer Biology, and bachelor’s degree in Biotechnology.